��Ŀ����
����Ϊ�����ṩ�˱������Ȼ��Դ��
��1��ʳ�ú�����ȡ����ҪӪ������ ��
��2����ͼΪ��ˮ����װ�����õ���Դ�� ����õ�����ˮ���� ������ĸ��ţ���
A������ B�������� C�������
��3���Ӻ�ˮ����ȡʳ�Σ����õķ����� ��
��4�����Ȼ��ƺ�̼����泥� �����Ʊ�̼�����ƺ��Ȼ�泥�
�����Ʊ�̼�����ƺ��Ȼ�泥� �����÷�Ӧ�ɱ�ʾΪ��
�����÷�Ӧ�ɱ�ʾΪ�� ��
��
20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100 gˮ�м��� g NaCl��
g NaCl�� g
g  �������ϴ���Һ���������������Ϊ g��
�������ϴ���Һ���������������Ϊ g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䡣
| ���� | NaCl |
|
|
|
| ��Һ��/g |
|
|
|
|
��1�������ʡ�����2��̫���ܡ�B������3�������ܼ��ᾧ������4��7.2g

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ϊ�����ṩ�˱������Ȼ��Դ��
��1��ʳ�ú�����ȡ����ҪӪ�������� ����
��2����ͼΪ��ˮ����װ�ã����õ���Դ���� ������õ�����ˮ������ ��������ĸ��ţ���

A������ B�������� C�������
��3���Ӻ�ˮ����ȡʳ�Σ����õķ������� ����
��4�����Ȼ��ƺ�̼����泥�NH4HCO3�����Ʊ�̼�����ƺ��Ȼ�泥�NH4Cl�����÷�Ӧ�ɱ�ʾΪ��NaCl+NH4HCO3�TNaHCO3+NH4Cl��
20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100gˮ�м���11.7g NaCl��15.8g NH4HCO3�������ϴ���Һ���������������Ϊ�� ��g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣮
|
���� |
NaCl |
NH4HCO3 |
NH4Cl |
NaHCO3 |
|
��Һ��/g |
36.0 |
21.6 |
37.2 |
9.6 |
����Ϊ�����ṩ�˱������Ȼ��Դ��
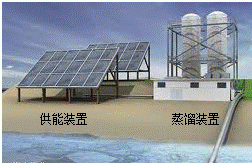
��1��ʳ�ú�����ȡ����ҪӪ������������
��2����ͼΪ��ˮ����װ�ã����õ���Դ����������õ�����ˮ��������������ĸ��ţ���
A������ B�������� C�������
��3���Ӻ�ˮ����ȡʳ�Σ����õķ�����������
��4�����Ȼ��ƺ�̼����泥�NH4HCO3�����Ʊ�̼�����ƺ��Ȼ�泥�NH4Cl�����÷�Ӧ�ɱ�ʾΪ��NaCl+NH4HCO3═NaHCO3+NH4Cl��
20��ʱ����������ѧ����ʽ�з�Ӧ��������ȣ���100gˮ�м���11.7g NaCl��15.8g NH4HCO3�������ϴ���Һ���������������Ϊ����g��
���ϣ�20��ʱ�������ʵ��ܽ�����£���������ͬʱ�ܽ���ˮ�и��Ե��ܽ�Ȳ��䣮
| ���� | NaCl | NH4HCO3 | NH4Cl | NaHCO3 |
| �ܽ��/g | 36.0 | 21.6 | 37.2 | 9.6 |





 ��2012?����������Ϊ�����ṩ�˱������Ȼ��Դ��
��2012?����������Ϊ�����ṩ�˱������Ȼ��Դ��
 �����Ʊ�̼�����ƺ��Ȼ�泥�
�����Ʊ�̼�����ƺ��Ȼ�泥� �����÷�Ӧ�ɱ�ʾΪ��
�����÷�Ӧ�ɱ�ʾΪ�� ��
��  g NaCl��
g NaCl�� g
g  �������ϴ���Һ���������������Ϊ g��
�������ϴ���Һ���������������Ϊ g��