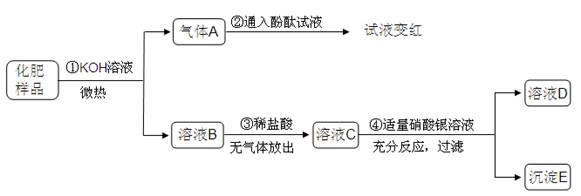
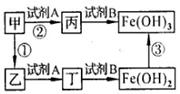
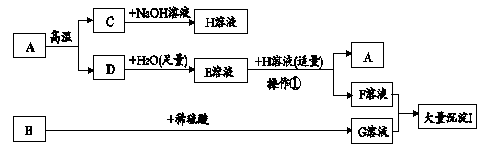
��Ŀ����
��10�֣�̼Ԫ�����γɻ�������������Ԫ�أ���̼��������ѧ��ѧ���о�����Ҫ���ݡ�
��1����ѧϰ��ѧ�Ĺ�����������ʶ�ģ��ٶ�����̼����̼��ơ�����֬�������͡��������ǣ�����̼����ij�Ա��������������Ϊ�����ṩ������Ӫ�������� ��ѡ���������ʵı����գ���
��2����ʯȼ���Dz���������Դ����ʯȼ����Ҫ����ú�� ����Ȼ�������Ƕ�����̼Ԫ�أ�д����Ȼ������Ҫ�ɷ���ȫȼ�յĻ�ѧ��Ӧ����ʽ ��
��3��С��ͬѧ��̼������Ҫ������֪ʶ���й��ɡ�����������������ͼ��ʾ��ת����ϵͼ��

��д��X�Ļ�ѧʽ______��
��д��ת����ϵͼ����Y��CO2�� һ����Ӧ��ѧ����ʽ ��
����̼�����ƣ�NaHCO3��������ȷֽ�Ҳ�ɵõ�CO2��ͬʱ������̼���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�Ļ���������_______��Ӧ��
��1����ѧϰ��ѧ�Ĺ�����������ʶ�ģ��ٶ�����̼����̼��ơ�����֬�������͡��������ǣ�����̼����ij�Ա��������������Ϊ�����ṩ������Ӫ�������� ��ѡ���������ʵı����գ���
��2����ʯȼ���Dz���������Դ����ʯȼ����Ҫ����ú�� ����Ȼ�������Ƕ�����̼Ԫ�أ�д����Ȼ������Ҫ�ɷ���ȫȼ�յĻ�ѧ��Ӧ����ʽ ��
��3��С��ͬѧ��̼������Ҫ������֪ʶ���й��ɡ�����������������ͼ��ʾ��ת����ϵͼ��

��д��X�Ļ�ѧʽ______��
��д��ת����ϵͼ����Y��CO2�� һ����Ӧ��ѧ����ʽ ��
����̼�����ƣ�NaHCO3��������ȷֽ�Ҳ�ɵõ�CO2��ͬʱ������̼���ƺ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�Ļ���������_______��Ӧ��
�� �ۢ� �� �� ʯ�ͣ� CH4 + 2O2 CO2 + 2H2O �� �� CO �� �� CaCO3
CO2 + 2H2O �� �� CO �� �� CaCO3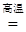 CaO+CO2��
CaO+CO2��
�� 2NaHCO3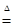 Na2CO3+H2O +CO2����2�֣� ���ֽ⣨1�֣�
Na2CO3+H2O +CO2����2�֣� ���ֽ⣨1�֣�
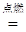 CO2 + 2H2O �� �� CO �� �� CaCO3
CO2 + 2H2O �� �� CO �� �� CaCO3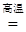 CaO+CO2��
CaO+CO2�� �� 2NaHCO3
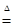 Na2CO3+H2O +CO2����2�֣� ���ֽ⣨1�֣�
Na2CO3+H2O +CO2����2�֣� ���ֽ⣨1�֣������������1����ѧϰ��ѧ�Ĺ�����������ʶ�ģ��ٶ�����̼����̼��ơ�����֬�������͡��������ǣ�����̼����ij�Ա�����ݸ���Ӫ���ص����ÿ�֪��������������Ϊ�����ṩ������Ӫ�������Т���֬���������� ��2����ʯȼ����Ҫ����ú��ʯ�ͺ���Ȼ�������Ƕ�����̼Ԫ�أ���Ȼ������Ҫ�ɷ��Ǽ��飬��ȫȼ�յĻ�ѧ��Ӧ����ʽCH4 + 2O2
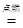 CO2 + 2H2O����3������ת����ϵ��֪�����е�XΪһ����̼��YΪ̼��ƣ��ʢ�X�Ļ�ѧʽΪCO����д��ת����ϵͼ����Y��CO2�� һ����Ӧ��ѧ����ʽCaCO3
CO2 + 2H2O����3������ת����ϵ��֪�����е�XΪһ����̼��YΪ̼��ƣ��ʢ�X�Ļ�ѧʽΪCO����д��ת����ϵͼ����Y��CO2�� һ����Ӧ��ѧ����ʽCaCO3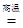 CaO+CO2��������̼�����ƣ�NaHCO3��������ȷֽ�Ҳ�ɵõ�CO2��ͬʱ������̼���ƺ�ˮ����ѧ����ʽΪ2NaHCO3
CaO+CO2��������̼�����ƣ�NaHCO3��������ȷֽ�Ҳ�ɵõ�CO2��ͬʱ������̼���ƺ�ˮ����ѧ����ʽΪ2NaHCO3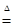 Na2CO3+H2O +CO2������Ӧ�Ļ�������Ϊ���ֽⷴӦ
Na2CO3+H2O +CO2������Ӧ�Ļ�������Ϊ���ֽⷴӦ
��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ