��Ŀ����
Ϊ�ⶨһƿ�����Ѿõ��ռ���NaOH������ijͬѧȡ�������ռ���Ʒ������һ������ˮ���õ�200g��Һ���ټ���200gϡ���ᣨ��������ֽ��赽���ٷų�����Ϊֹ��������ҺΪ395.6g��������ش�
��1����Ӧ������CO2������Ϊ______g��
��2������ȡ��ƷΪ50.0g������Ʒ��NaOH�����������Ƕ��٣���д��������̣�
��3������������ȵ��ռ����ݣ�����һ��ʱ�����һ��δ���ʡ�һ�ݲ��ֱ��ʡ�һ��ȫ�����ʣ�ע�����ʺ�����ʶ�ΪNa2CO3�������ֱ�����ͬ���������������ַ�Ӧʱ����Ҫ�����������ȣ���ԭ����______��
�⣺��1�����������غ㶨�ɣ���Ӧ������CO2������=200g+200g-395.6g=4.4g
�ʴ�Ϊ��4.4��
��2������ȡ��Ʒ�к���Na2CO3������Ϊx
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
106 44
x 4.4g
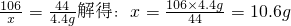
��Ʒ��NaOH����������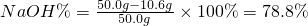
���ռ���Ʒ��NaOH����������Ϊ78.8%
��3�����ݷ�Ӧǰ��Ԫ���������䣬���ʵ�������������Ԫ���������䣬�����ᷴӦ���������ͬ�����������ƣ����е���������������������������ȫ���������������ϡ���ᣬ��ˣ�����ϡ���������Ҳ��ȣ�
�ʴ�Ϊ��������Ԫ�������غ��ϵ���������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�ͬʱ����98g���ᣮ
��������1�����������غ㶨�ɣ����ݷ�Ӧǰ�������������IJ�ɼ���ų�������̼��������
��2��������ȫ��Ӧ�ų�������̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ������Ʒ��̼���Ƶ�����������Ʒ������̼���������IJ�����Ʒ������ȣ������Ʒ��NaOH������������
��3�����ݷ�Ӧǰ��Ԫ���������䣬���ʵ�������������Ԫ���������䣬�����ᷴӦ���������ͬ�����������ƣ����������������ȫ���������������ϡ���ᣬ��ˣ��ɵõ�������Ʒ���ĵ���ϡ����Ľ��ۣ�
���������������غ㶨�ɼ������Ӧ���ų�������̼�������ǽ���������㣮
�ʴ�Ϊ��4.4��
��2������ȡ��Ʒ�к���Na2CO3������Ϊx
Na2CO3+H2SO4=Na2SO4+H2O+CO2��
106 44
x 4.4g
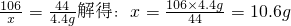
��Ʒ��NaOH����������
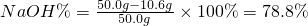
���ռ���Ʒ��NaOH����������Ϊ78.8%
��3�����ݷ�Ӧǰ��Ԫ���������䣬���ʵ�������������Ԫ���������䣬�����ᷴӦ���������ͬ�����������ƣ����е���������������������������ȫ���������������ϡ���ᣬ��ˣ�����ϡ���������Ҳ��ȣ�
�ʴ�Ϊ��������Ԫ�������غ��ϵ���������ƺ�̼���ƶ���ÿ46g��Ԫ������142g�����ƣ�ͬʱ����98g���ᣮ
��������1�����������غ㶨�ɣ����ݷ�Ӧǰ�������������IJ�ɼ���ų�������̼��������
��2��������ȫ��Ӧ�ų�������̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ������Ʒ��̼���Ƶ�����������Ʒ������̼���������IJ�����Ʒ������ȣ������Ʒ��NaOH������������
��3�����ݷ�Ӧǰ��Ԫ���������䣬���ʵ�������������Ԫ���������䣬�����ᷴӦ���������ͬ�����������ƣ����������������ȫ���������������ϡ���ᣬ��ˣ��ɵõ�������Ʒ���ĵ���ϡ����Ľ��ۣ�
���������������غ㶨�ɼ������Ӧ���ų�������̼�������ǽ���������㣮

��ϰ��ϵ�д�
�����Ŀ