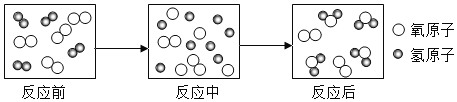
��Ŀ����
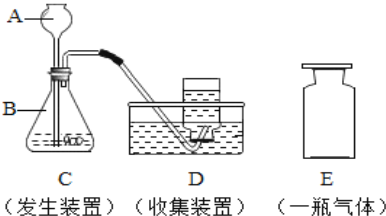
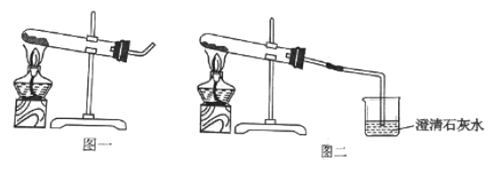
����Ŀ��ij��ȤС���KClO3�ֽⷴӦ�Ĵ��������о�������ͬ�ļ��������£�����ͼװ����ɱ���ʵ��:
��� | KClO3����/g | ���� | ��������/g | �ռ�50mLO2����ʱ��/s |
ʵ��1 | 5 | - | - | 171 |
ʵ��2 | 5 | MnO2 | 0.5 | 49 |
ʵ��3 | 5 | Fe2O3 | 0.5 | 58 |
ʵ��4 | 5 | KCl | 0.5 | 154 |
��1������ʵ��1��Ŀ����___________________
��2����������3�ִ����Ĵ�Ч����ѵ���______________
��3����ʵ��1��ʵ��4��֪��KCl____�������������������������á�ά�ּ����������䣬��ʵ��1�ټ����ռ��ռ�50mLO2������ʱ����������171s������ԭ��______________��
��4��Ҫ�Ƚ�KClO3�ֽⷴӦ�в�ͬ�����Ĵ�Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ����________________��
���𰸡��Ա�ʵ�� MnO2 �� ���ɵ�KCl�ӿ��˷�Ӧ �ռ���������
��������
��1��ͨ���ԱȲ���֪��ʵ��2��3��4�м���ҩƷ���������ã�
��2�����˼���ҩƷ��ͬ�⣬������������ͬ�����ݶԱȿ�֪���ռ���ͬ�����������ʵ��2����ʱ����̣����������̵Ĵ�Ч����ã�
��3����ʵ��1��ʵ��4��֪��KCl�д����ã�ά�ּ����������䣬��ʵ��1�ټ����ռ��ռ�50mLO2������ʱ����������171s������Ϊ����طֽ����ɵ��Ȼ��ضԷ�Ӧ���˴����ã�
��4��Ҫ�Ƚ�KClO3�ֽⷴӦ�в�ͬ�����Ĵ�Ч�������˲����ռ�50mLO2����ʱ���⣬�����Բ�����ͬʱ�����ռ������������
�ʴ�Ϊ��
��1���Ա�ʵ�飻
��2��MnO2��
��3���У����ɵ�KCl�ӿ��˷�Ӧ��
��4���ռ�����������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����СӢ�ҵIJֿ���ѷ���һ��������һһ̼�����( NH4HCO3).����һ�����죬СӢ�������ֻ��������еĴ̼�����ζ��ø�Ũ���ˣ���Щ���ʴ���̼����隣����ˣ���鷢�ֱ��ٵĻ��ʰ�װ��û���ܷ⣬����Ҳû�������ڵ��ϣ���û���˽����ֿ��ʹ�á�
Ϊ��̽����Щ���ʼ��ٵ�ԭ��СӢ��ʵ����ȡ��һЩ̼����立�ĩ�������������м��ȣ���һ����۲쵽��ĩ��ȫ��ʧ��ͬʱҲ�ŵ������ִ̼�����ζ.��ĩΪʲô����ʧ��?
(1)��������⣩̼����立�ĩ��ʧ��ԭ����ʲô?
(2)�����룩��̼����立�ĩ�ڲ����Ȼ�����������ɹ�̬���������̬����̼������ڲ����Ȼ���������·����ֽⷴӦ�����ܲ����������а�����һЩ�����
(3)���������ϣ���̼��������ڰ��ʣ����������������ʣ�˵����������__________ (�����)���������ڰ���(��ѧʽNH3)��������Ĵ̼�����ζ����������ˮ����ˮ��Һ�Ǽ��ԣ�������İ�������ʹ����ĺ�ɫʯ���Լ���������NO2Ϊ����ɫ���塣NOΪ��ɫ���壬�ڿ�����������Ӧ��2NO+O2=2NO2
(4)��ʵ���������������ۣ�
ʵ����� | ʵ������ | ʵ����� |
��ȡ����̼��������Թ��м��ȣ���ͼһ��ʾ��������ĺ�ɫʯ����ֽ�ӽ����ܿ� | ����ǿ�ҵĴ̼�����ζ���Թܱ�������ɫҺ������ֽ��������δ������ɫ���� | �ֽ��������______��û��__________ |
�ڰ���ͼ����ʾװ�ü���ʵ�飬ֱ����Ӧ��ȫ | ����ʯ��ˮ����� | �ֽ��������__________ |
(5)��Ӧ�ã������ð����Ļ�ѧ���ʣ���д��ʵ���Ҽ��鰱���ķ���(д��ʵ�������������)��________________________________________��
������������̼����炙��ʣ���Ӧ����α���? ______________________________��
����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
��������⣩������ͭ�Ƿ�Ҳ��������طֽ�Ĵ������Ƿ�ȶ������̴�Ч�����ã�
����Ʋ����ʵ�飩
I���� 3.0g ����ط����Թ��м���
II���� 3.0g ������� 1.0g �������̾��Ȼ�ϼ���
III���� Xg ������� 1.0g ����ͭ���Ȼ�ϼ���
��ʵ�����������III �� X ��ֵӦΪ_____����ʵ�� I �� III �ȽϿ�֤��_____��
��ʵ�鷴Ӧ��Ĺ����ˮ�ܽ⡢���ˡ�ϴ�ӡ�����������õ� 1.0g ��ɫ��ĩ���ٽ���ɫ��ĩ�� Xg ����ػ�ϼ��ȣ� ������ʵ�� III ��ͬ���˲�����Ϊ��֤������ͭ�ڸû�ѧ��Ӧǰ��_____��_____�����䣻
�����ۣ�����ͭҲ��������طֽ�Ĵ�����
��ʵ�鷴˼��ʵ�� II �� III �Ա���Ϊ��֤��_____��
��������⣩��˫��ˮ�ֽ��ٶȻ���ʲô�����йء�
����Ʋ����ʵ�飩
ʵ�� | ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2 ������ | ��ͬʱ���ڲ��� O2 ��� |
1 | 50.0g | 1% | 0.1g | 9mL |
2 | 50.0g | 2% | 0.1g | 16mL |
3 | 50.0g | 4% | 0.1g | 31mL |
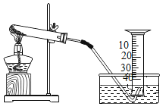
��ʵ���У����� O2 �����װ����_____�����ţ���
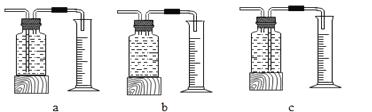
�����ۣ������ϱ���֪������ͬ�����£�˫��ˮ��Ũ��Խ��˫��ˮ�ֽ������Խ_____��