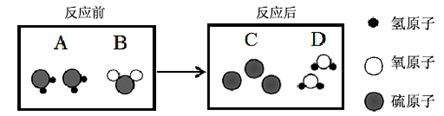
��Ŀ����
���ұ�����ʵ��С��ֱ���С�Na2CO3��NaOH�������Na2CO3�����ⶨ����ʵ�飺
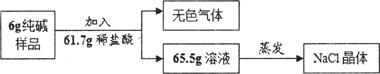
(1)�����ʵ�鷽���ǣ���50�˵Ļ�����ܽ���ˮ�������Һ���μ�10%ϡ���ᣬ�۲쵽���� ����������������Һ���������ⶨ̼���Ƶĺ���������Ϊ��������Ƿ���ȷ,������_______ ��
��2������ͬѧ�ķ����ǣ����ݳ�������������ó�̼���Ƶĺ�����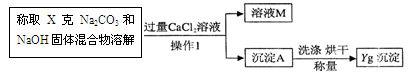
�Ҿ���ȷ���㣬�ó�̼���Ƶĺ���ƫ��ԭ������� ����ҺM�е����ʣ��û�ѧʽ��ʾ���� ��
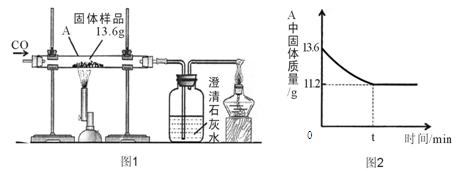
��3�������ʵ�鷽���ǣ���50����Ʒ��������ϡ���ᷴӦ������ͼװ�òⶨ������CO2����������ͨ������ó���Ʒ��Na2CO3�������������װ�����Ͳ��������____ _ ____�����ռ���0��1Ħ��CO2���壬��ԭ�������Na2CO3�������ٷ����Ƕ��٣�����ȷ��0��1%���������ݻ�ѧ����ʽ��д��������̣�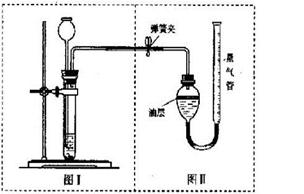
��1����1����һ����������� ��1�֣�����ȷ����Ϊ̼���ƺ��������ƶ����������� ��1�֣���2��ϴ�Ӳ���ֻ�û����ȫ����(1��)�� (1��)�Ȼ��ơ�(1��)�Ȼ��ƺ�(1��)�������ƣ�3����ֹ������̼�ܽ���ˮ�л��ˮ��Ӧ �� 21��2%
����

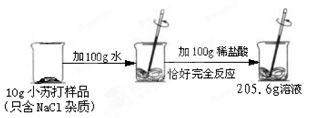
��7�֣�ѧ��������Ժ���˼ͬѧͨ��ʵ��̽��п����������������������������Һ�ķ�Ӧ��ʵ��������������£�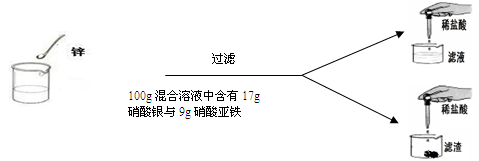
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �����������������Ļ����Һ | 100g | 100g | 100g | 100g |
| � | 2g | 3.25g | m | 9.75g |
| ����Һ�м���ϡ������ʵ������ | ������ɫ���� | ���������� | ���������� | ���������� |
| �������м���100 gϡ������ʵ������ | ���������� | ���������� | �������ݣ���Һ��Ϊdz��ɫ | �������������ͬ |
��2�����ݵ�һ�ε�ʵ����������Ϊ�˴���Һ�е������� ��
��3��������֪�����г����ڶ���ʵ�����û���������������(x)�ı���ʽ ��
��4����������ʵ��п����������Һǡ����ȫ��Ӧ����m������Ϊ__________________��
��5����������η�Ӧ�����Һ�м���һ��������ˮ�����ò�������Һ�����ʵ���������Ϊ10%�������ˮ������Ϊ__________________��
��6������������������Ϊ36.5����Ũ�������Ƶ��Ĵ�ʵ����������ǡ����ȫ��Ӧ�����ϡ���ᣬ����ҪŨ������ˮ��������Ϊ_______________��
(7��))����ʱ��ͬѧ�Dz����������ַ����ⶨij�Ȼ�����Һ����������������
��1������ѧ��������һ�����Ȼ�����Һ�м���������������Һ���õ�2.87g�Ȼ������壬����Ȼ�����Һ���Ȼ��Ƶ�����Ϊ���٣�(���ݻ�ѧ����ʽ��ʽ����)
�����ʵ��ⶨ������Һ��������������Ϊ10%��
��2��������������ȡһ��������Һ��������������ʵ���������£�
| �����������(g) | 25.0 |
| ������+ʳ����Һ(g) | 45.0 |
| ������+ʳ�ξ���(g) | 27.4 |
| ���ݴ��� | ��Һ��������������Ϊ |
A������ʱδ�ò���������
B����ȡ�Ȼ�����Һ�����ϴ�
C������ʱ������������ʱ��ֹͣ����
D��ʵ���δ���������ϵİ�ɫ��������������