��Ŀ����
����һ������ˮ��Һ��ֻ���ܺ������������е����ɣ�Na+��NH4+��Ba2+��Cl-��CO32-��ij��ȤС���ͬѧһ����ƣ������������ӵĴ��ڣ���1����ͬѧ˵�������ü���Һ����NH4+����ͬѧ����Һ�еμ�NaOH��Һ�ȣ��ŵ���һ�ɴ̼�����ζ��Ȼ��һ��ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ��
��2����ΪBaCO3
��3����ͬѧ�ֱ�ȡ����200mL��Һ��������ʵ�飺
��
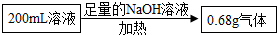 �ɴ˼��㣺200mL��Һ�к�NH4+������Ϊ
�ɴ˼��㣺200mL��Һ�к�NH4+������Ϊ��
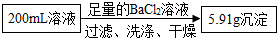 �ɴ˼��㣺200mL��Һ�к�CO32-������Ϊ
�ɴ˼��㣺200mL��Һ�к�CO32-������Ϊ�۸���Һ��NH4+��CO32-�ĸ�����Ϊ
��������1������������
��2��Ba2+��CO32-��Ӧ���ɰ�ɫ����
��3������Һ�У������������������������ȣ�
��2��Ba2+��CO32-��Ӧ���ɰ�ɫ����
��3������Һ�У������������������������ȣ�
����⣺��1�����μ�NaOH��Һ�ȣ��ŵ���һ�ɴ̼�����ζ��Ȼ�����һ��ʪ��ĺ�ɫʯ����ֽ���Թܿڣ���ֽ������
�ʴ�Ϊ������NH4Cl+NaOH�TNaCl+NH3��+H2O
��2������Һ�еμ�BaCl2��Һ���а�ɫ�������֣�˵����Һ��һ������̼������ӣ���ԭ��Һ��һ�����������ӣ�
�ʴ�Ϊ������Ba2+
��3����������������NH4Cl+NaOH�TNaCl+NH3��+H2O��
֪������������Ϊ0.68�ˣ��������笠����ӵ�������
��100%=0.72�ˣ�
�ʴ�Ϊ��0.72
��Ba2+��CO32-��Ӧ���ɰ�ɫ������Ba2++CO32=BaCO3��֪����ɫ������������5.91�ˣ���������̼������ӵ�������
��100%=1.8�ˣ�
�ʴ�Ϊ��1.8
��笠�������̼������ӵĸ���֮��Ϊ����0.72��18������1.8��60��=4��3��
�ʴ�Ϊ��4��3
����Һ�У������������������������ȣ�
�ʴ�Ϊ��һ��������
�ʴ�Ϊ������NH4Cl+NaOH�TNaCl+NH3��+H2O
��2������Һ�еμ�BaCl2��Һ���а�ɫ�������֣�˵����Һ��һ������̼������ӣ���ԭ��Һ��һ�����������ӣ�
�ʴ�Ϊ������Ba2+
��3����������������NH4Cl+NaOH�TNaCl+NH3��+H2O��
֪������������Ϊ0.68�ˣ��������笠����ӵ�������
| 53.5��0.68�ˡ�18 |
| 17��53.5 |
�ʴ�Ϊ��0.72
��Ba2+��CO32-��Ӧ���ɰ�ɫ������Ba2++CO32=BaCO3��֪����ɫ������������5.91�ˣ���������̼������ӵ�������
| 5.91��60 |
| 197 |
�ʴ�Ϊ��1.8
��笠�������̼������ӵĸ���֮��Ϊ����0.72��18������1.8��60��=4��3��
�ʴ�Ϊ��4��3
����Һ�У������������������������ȣ�
�ʴ�Ϊ��һ��������
����������Һ�У�����Ҫ�빲�棬��䲻�ܷ�����Ӧ�������������塢ˮ���������ɣ�

��ϰ��ϵ�д�
�����Ŀ