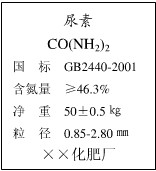
��Ŀ����
��3�֣���ͭ����ͭ��п����ɵĺϽ���;�㷺��ij��ȤС��Ϊ̽����ͭ�Ͻ����ɣ�ȡ20.00 g��ĩ״��ͭ�Ͻ���Ʒ����60.00 gϡ����ƽ���ֳ����ȷݣ������μ�����Ʒ�У����������������ַ�Ӧ���˳����壬����ϴ�ӡ������������ʵ���������£�
����㣺��Ҫ��д��������̣�
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ����ͼ��ʾ��������BaCl2��Һ����������������
| | ��һ�� | �ڶ��� | ������ |
| ʣ������������g | 16.75 | 13.50 | 12.40 |
��1���û�ͭ�Ͻ��У�пԪ�ص�����������
��2���ڶ���ʵ���зų������������
��3��������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ���������ó�����������BaCl2��Һ�����Ĺ�ϵ����ͼ��ʾ��������BaCl2��Һ����������������

��1��38% ��2��0.1 g ��3��15%
�����������1��ͭ��п����ɵĺͽ��У�п��ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ�����ݱ����е����ݿ�֪��ǰ���Σ���ÿ�μ���20gϡ����ʱ����������������20g-16.75g=3.25g��˵��ÿ��п����Ӧ��3.25g�����˱�������ô�����Σ�Ӧ��ʣ���������Ϊ13.50g-3.25g=10.25g�����ɱ����е����ε����ݿ�֪��ʣ���������Ϊ12.40g��˵����ʱ��п����ȫ��Ӧ��ʣ��Ĺ���Ϊ����Ӧ��ͭ�������ɱ����֪ͭ������Ϊ12.40g����п������=20.00g-12.40g=7.60g����пԪ�ص���������=
 ��100%=
��100%=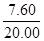 ��100%=38%��
��100%=38%����2���ӱ����п�֪����һ�κ͵ڶ���֮���������˵�����������20gϡ���ᷴӦ��п���������ɴ˸��ݻ�ѧ����ʽ����֪�ķ�Ӧ�����������������ɵ������������
�ɱ����֪����һ��ʣ�����16.75g���ڶ���Ϊ13.50g��˵���ڶ��η�Ӧ��п������Ϊ16.75-13.50=3.25g��
��ڶ���ʵ���зų����������ΪX����������ã�
Zn+H2SO4=ZnSO4+H2��
65 2
3.25g X
65��2 =3.25g��Xg
��ã�X=0.1g
��3���������ʵ������û����Һ�еμ�BaCl2��Һ�����ɲ�����ˮ��BaSO4��ɫ��������ʵ���DZ����Ӻ���������ӽ�ϣ��������ᱵ�����������������������ϡ���ᣬ�ʴ˳����൱���������Ȼ�����Ӧ���ɡ���ֻҪ���������������Ϳ�������Ȼ�����������
���ڵ�һ�κ͵ڶ��η�Ӧʱ����������ȫ��Ӧ�����ɴ����20g�����е�����������
��20g�����е���������Ϊy
Zn + H2SO4 = ZnSO4 + H2��
65 98
3.25g y
65��98=3.25g��y
���y=4.9g
��20g��������ʵ���������=
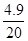 ��100%=24.5%
��100%=24.5%��ô60.00g���������ʵ�����=60.00��24.5%=14.7g
����60.00g������ȫ��Ӧ����Ҫ�Ȼ���������Ϊz
H2SO4+BaCl2=BaSO4��+2HCl
98 208
14.7g z
98��208=14.7g��z
��ã�z=31.2g
���ݡ����ó�����������BaCl2��Һ�����Ĺ�ϵͼ��ʾ�������õ�BaCl2��Һ������Ϊ208�ˣ���BaCl2��Һ��������������=
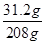 ��100%=15%
��100%=15%�𣺣�1���û�ͭ�Ͻ��У�пԪ�ص���������38%����2���ڶ���ʵ���зų����������0.1g��
��3��BaCl2��Һ��������������15%��
���������⿼����ͼʾ�ͻ�ѧ��Ӧ���ϵ�ͨ����ѧ����ʽ���м��㣬�����Ѷ��ϴ����Ĺؼ����ܹ��ӱ������ҳ�������ϵ�����շ�Ӧ���������ѧ����ͼ������ͼʾ������Ҫ��ϸߡ�

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ