��Ŀ����
ʯ��ʯ�DZ��ص���Ҫ�����Դ�����ijˮ�೧�Ļ���ԱΪ�ⶨ�չ�����һ��ʯ��ʯ��̼��Ƶ������������������������ⶨijʯ��ʯ��Ʒ�Ĵ��ȣ��䷴Ӧԭ�������ʲ������ᷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2����ʵ��ʱ���Ƚ�ʢ������ϡ�������ƿ����������֮��Ϊ300.0g�������ڵ�����ƽ�ϣ��ٽ�25.0g��ʯ��ʯ������ƿ�У�����������Ӧ��ÿ��3���ֶ��õ�����ƽ���������£�

��1��ʵ���У���ƿ�ڷ�һС����������Ҫ������
��2��������M��ֵ��
��3������ʵ������������Ӧʱ�������������ʵ��������٣���m����Ĺ�ϵ��ͼ��
��4�������ʯ��ʯ��Ʒ��̼��Ƶ�����������

| ��Ӧ��ʱ�䣨min�� | 3 | 6 | 9 | 12 | 15 |
| ������ƽ������g�� | 320.6 | 317.3 | 315.1 | M | 315.1 |
��ֹ�����е����ʽ�����ƿ
��ֹ�����е����ʽ�����ƿ
����2��������M��ֵ��
315.1
315.1
g������3������ʵ������������Ӧʱ�������������ʵ��������٣���m����Ĺ�ϵ��ͼ��
��4�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��������1��������ƿ�ڷ�һС����������Ҫ�����Ƿ�ֹ�����е����ʽ�����ƿ���н��
��2��������ͼ����������9min��15minʱ��������ͬ��˵��9minʱ��Ӧ����ɣ���ô12minʱ������Ӧ�ú�9min��15minʱ��һ�����н��
��3�����������غ㶨�ɵ�Ӧ�öԷ�Ӧ�������ļ���ֵ���зֱ���м��㣬�����ͬʱ���ж�����̼����ֵ������������㷨����ͼ�ɣ�
��4�����ݻ�ѧ����ʽ���������������֮��������ȣ��������̼��Ƶ��������ٸ�������������ʽ�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
��2��������ͼ����������9min��15minʱ��������ͬ��˵��9minʱ��Ӧ����ɣ���ô12minʱ������Ӧ�ú�9min��15minʱ��һ�����н��
��3�����������غ㶨�ɵ�Ӧ�öԷ�Ӧ�������ļ���ֵ���зֱ���м��㣬�����ͬʱ���ж�����̼����ֵ������������㷨����ͼ�ɣ�
��4�����ݻ�ѧ����ʽ���������������֮��������ȣ��������̼��Ƶ��������ٸ�������������ʽ�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
����⣺��1����ƿ�ڷ�һС����������Ҫ�����Ƿ�ֹ�����е����ʽ�����ƿ��Ӱ��ʵ�����������ֹ�����е����ʽ�����ƿ��
��2����ͼ����������9min��15minʱ��������ͬ��˵��9minʱ��Ӧ����ɣ���ô12minʱ������Ӧ�ú�9min��15minʱ��һ�����ʱ���M��ֵӦ����315.1�����315.1��
��3�����ڷ�Ӧ�����ʵļ���ֵΪ������̼�����������Կɵõ���ͬʱ������ɶ�����̼�����������Ӧ��ϵ�����ʾ��
������㷨�ɵ�ͼ����ͼ��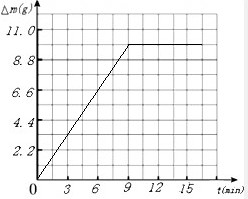 �����
����� ��
��
��3�����շ�Ӧ���ɶ�����̼������Ϊ��325g-315.1=9.9g
��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 9.9g
=
x=22.5g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��
��100%=90%
�𣺣�4����ʯ��ʯ��̼��Ƶ���������Ϊ90%��
��2����ͼ����������9min��15minʱ��������ͬ��˵��9minʱ��Ӧ����ɣ���ô12minʱ������Ӧ�ú�9min��15minʱ��һ�����ʱ���M��ֵӦ����315.1�����315.1��
��3�����ڷ�Ӧ�����ʵļ���ֵΪ������̼�����������Կɵõ���ͬʱ������ɶ�����̼�����������Ӧ��ϵ�����ʾ��
| ��Ӧ��ʱ�䣨min�� | 3 | 6 | 9 | 12 | 15 |
| ������̼��������g�� | 4.4 | 7.7 | 9.9 | 9.9 | 9.9 |
 �����
����� ��
����3�����շ�Ӧ���ɶ�����̼������Ϊ��325g-315.1=9.9g
��ʯ��ʯ��Ʒ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 9.9g
| 100 |
| x |
| 44 |
| 9.9g |
x=22.5g
ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ��
| 22.5g |
| 25 |
�𣺣�4����ʯ��ʯ��̼��Ƶ���������Ϊ90%��
�����������Ĺؼ��Ǹ��������غ㶨�ɣ�ȷ�жϳ�������̼�ͷ�Ӧ�����ɵ���Һ��������

��ϰ��ϵ�д�
�����Ŀ
ʯ��ʯ�DZ��ص���Ҫ�����Դ�����ijˮ�೧�Ļ���ԱΪ�ⶨ�չ�����һ��ʯ��ʯ��̼��Ƶ������������������������ⶨijʯ��ʯ��Ʒ�Ĵ��ȣ��䷴Ӧԭ�������ʲ������ᷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2����ʵ��ʱ���Ƚ�ʢ������ϡ�������ƿ����������֮��Ϊ300.0g�������ڵ�����ƽ�ϣ��ٽ�25.0g��ʯ��ʯ������ƿ�У�����������Ӧ��ÿ��3���ֶ��õ�����ƽ���������£�
| ��Ӧ��ʱ�䣨min�� | 3 | 6 | 9 | 12 | 15 |
| ������ƽ������g�� | 320.6 | 317.3 | 315.1 | M | 315.1 |
��2��������M��ֵ��______g����
��3������ʵ������������Ӧʱ�������������ʵ��������٣���m����Ĺ�ϵ��ͼ��
��4�������ʯ��ʯ��Ʒ��̼��Ƶ�����������
 ʯ��ʯ�����е���Ҫ���֮һ��Ϊ�˲ⶨ����ʯ��ʯ��̼��Ƶ�����������ijѧУ�о���ѧϰС�����������ʵ�飺ȡʯ��ʯ��Ʒ25g�������еμ�ϡ���ᣬ�ⶨ��������CO2������������ϡ��������������CO2�����������ϵ��ͼ��ʾ���Լ��㣨���ʲ����ᷴӦ������Ҳ���ֽ⣩��
ʯ��ʯ�����е���Ҫ���֮һ��Ϊ�˲ⶨ����ʯ��ʯ��̼��Ƶ�����������ijѧУ�о���ѧϰС�����������ʵ�飺ȡʯ��ʯ��Ʒ25g�������еμ�ϡ���ᣬ�ⶨ��������CO2������������ϡ��������������CO2�����������ϵ��ͼ��ʾ���Լ��㣨���ʲ����ᷴӦ������Ҳ���ֽ⣩��