��Ŀ����
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��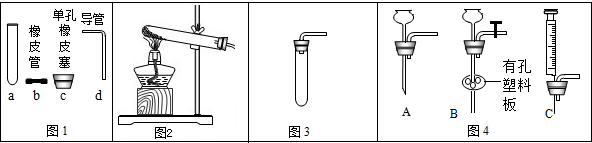
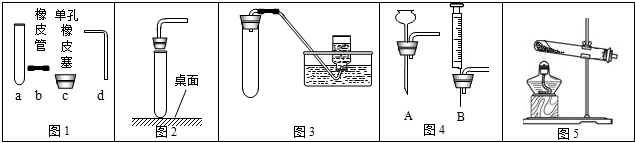
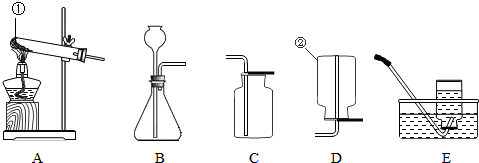
��1������ͼ1�ش�������c��dʱ��ʹd���ײ���c�еĴ�ʩ��
��2������ͼ2��ʾװ����ȡ�����Ļ�ѧ����ʽΪ
��3������ͼ3��ʾװ�ã��г�����ʡ�ԣ�������ȡ����
���ѧʽ�����䷴Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ
��4��ͼ3�����巢��װ����Ȼ���������㣬�������Ʒ�Ӧ���ʣ�Ҳ����ʱ���Ʒ�Ӧ�ķ�����ֹͣ������ͼ4��ѡȡװ��
��5��ij��ȤС�����Ȼ�粒�����������ƹ�����ȷ�Ӧ��ȡ��������ѡ��ͼ
��������1�����յ�������Ƥ�ܵ����ӷ�����
��2����ȷ��дͼ2��ʾװ����ȡ�����Ļ�ѧ����ʽ��
��3��̽��ͼ3��ʾװ�ÿ�����ȡ�����弰�䷴Ӧ�Ļ�ѧ����ʽ��
��4��̽��ͼ3�����巢��װ�ü��ܿ��Ʒ�Ӧ���ʣ�������ʱ���Ʒ�Ӧ�ķ�����ֹͣ�ķ�����
��5��̽���̹̼����ͷ�Ӧװ�ã�
��2����ȷ��дͼ2��ʾװ����ȡ�����Ļ�ѧ����ʽ��
��3��̽��ͼ3��ʾװ�ÿ�����ȡ�����弰�䷴Ӧ�Ļ�ѧ����ʽ��
��4��̽��ͼ3�����巢��װ�ü��ܿ��Ʒ�Ӧ���ʣ�������ʱ���Ʒ�Ӧ�ķ�����ֹͣ�ķ�����
��5��̽���̹̼����ͷ�Ӧװ�ã�
����⣺��1������ͼ1�ش�������c��dʱ��ʹd���ײ���c�еĴ�ʩ�ǣ���������Ƥ���ĵ���һ������ˮ��ʪ��
��2������ͼ2��ʾװ����ȡ����������û�����ţ����Բ����ø��������ȡ����������أ���ѧ����ʽΪ��2KClO3
2KCl+3O2������
��3������ͼ3��ʾװ�ã��г�����ʡ�ԣ�������ȡ�����ǣ�O2��CO2����H2����
�䷴Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��2H2O2
2H2O+O2����CaCO3+2HCl�TCaCl2+H2O+CO2������Zn+H2SO4�TZnSO4+H2������
��4��ͼ3�����巢��װ����Ȼ���������㣬�������Ʒ�Ӧ���ʣ�Ҳ����ʱ���Ʒ�Ӧ�ķ�����ֹͣ������ͼ4��ѡȡװ�� C ��ͼ1������a��װ���µ����巢��װ�ã���ɿ��Ʒ�Ӧ�����ʣ�����ͼ4��ѡȡװ�� B ��ͼ1������a��װ���µ����巢��װ�ã������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��5�����Ȼ�粒�����������ƹ�����ȷ�Ӧ��ȡ���������ڹ̹̼����ͷ�Ӧװ�ÿ�ѡ��ͼ 2�е����巢��װ�ã�
�ʴ�Ϊ����1����������Ƥ���ĵ���һ������ˮ��ʪ��
��2��2KClO3
2KCl+3O2����
��3��O2��CO2����H2����2H2O2
2H2O+O2����CaCO3+2HCl�TCaCl2+H2O+CO2������Zn+H2SO4�TZnSO4+H2������
��4��C��B��
��5��2��
��2������ͼ2��ʾװ����ȡ����������û�����ţ����Բ����ø��������ȡ����������أ���ѧ����ʽΪ��2KClO3
| ||
| �� |
��3������ͼ3��ʾװ�ã��г�����ʡ�ԣ�������ȡ�����ǣ�O2��CO2����H2����
�䷴Ӧ�Ļ�ѧ����ʽ�ֱ�Ϊ��2H2O2
| ||
��4��ͼ3�����巢��װ����Ȼ���������㣬�������Ʒ�Ӧ���ʣ�Ҳ����ʱ���Ʒ�Ӧ�ķ�����ֹͣ������ͼ4��ѡȡװ�� C ��ͼ1������a��װ���µ����巢��װ�ã���ɿ��Ʒ�Ӧ�����ʣ�����ͼ4��ѡȡװ�� B ��ͼ1������a��װ���µ����巢��װ�ã������ʱ���Ʒ�Ӧ�ķ�����ֹͣ��
��5�����Ȼ�粒�����������ƹ�����ȷ�Ӧ��ȡ���������ڹ̹̼����ͷ�Ӧװ�ÿ�ѡ��ͼ 2�е����巢��װ�ã�
�ʴ�Ϊ����1����������Ƥ���ĵ���һ������ˮ��ʪ��
��2��2KClO3
| ||
| �� |
��3��O2��CO2����H2����2H2O2
| ||
��4��C��B��
��5��2��
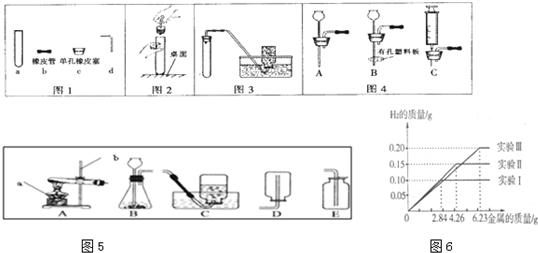
�������������ʵ�������ע�������ѧ����ʽ����д��ʵ��װ�õ�ѡ�õȷ����֪ʶ���ȶ�����ѧ����ѧ����ʽ����д���ֶ�����ѧ����ʵ��װ��ѡ�õ�̽������������������壮

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
 20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�
20����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�