��Ŀ����
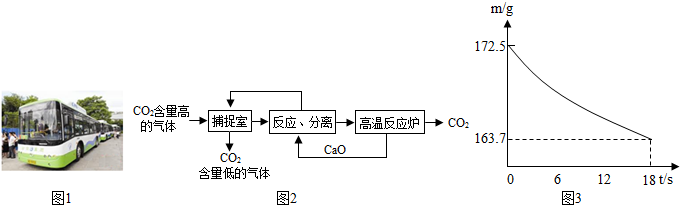
��̼����������ָͨ��һ���ķ���������ҵ�����в�����CO2���벢���д�������á�
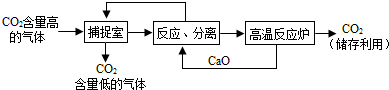
������NaOH��Һ��������CO2��������������ͼ��ʾ����������������δ�������
�� ���� �·�Ӧ¯���������CO2���Ƴɸɱ����ɱ������� ��24�� ��
�·�Ӧ¯���������CO2���Ƴɸɱ����ɱ������� ��24�� ��
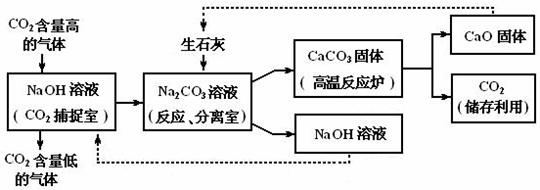
�� ����Ӧ�������ҡ��з�Ӧ������NaOH��CaCO3����IJ��������� ��25�� ��
�� ��CO2���ҡ��з����Ļ�ѧ��ӦΪ��CO2+2NaOH��Na2CO3+H2O���漰���������У�ˮ��Һ�ʼ��ԣ���ʹ��ɫ��̪��Һ���ɫ�������� ��26�� ����д��ѧʽ����
�� �����йظò����̵�������ȷ���� ��27�� ���ɶ�ѡ��
A��������CO2�����Ʊ�����������Ʒ�����������������ŷ�
B�����������У�ֻ��һ�����ʿ�ѭ������
C������Ӧ�����ҡ��еķ�Ӧ�ų���������
D���ò��������ŵ���û��������Դ
| �˹����꣨������������ʳƷ�����ȣ� | ||
| 25��25�� | ���� | |
| 25��26�� | NaOH��Na2CO3������2�֣� | ȫ�Ը�2�֣��ж��д���1�� |
| 25��27�� | A��C������2 | һ��һ��1�֣�����һ��1�֣�ȫѡ0�֣� |

��ϰ��ϵ�д�
�����Ŀ
 �֣�
�֣�