��Ŀ����
��ˮ�Ǿ����Դ���⡣��ͼ��ʾ���ú�ˮΪԭ�Ͽɻ���������Ʒ��
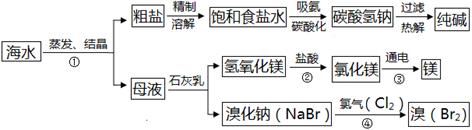
��1���������ѡ�������ᾧ�������ý��½ᾧ���������� ��
��2���ƴ�������У����й��˲�������Ҫ�IJ����������ձ���©���� ��
��3������ڷ�����Ӧ�Ļ�ѧ����ʽ�� ���������Ӧ������ ����þ�����һ��þ���Ͻ����������ɻ���ǵIJ��ϣ��ò��Ͼ��е���������
�� ���δ�һ�㣩��
��4������ܷ����ķ�ӦΪ�û���Ӧ�������ڽ���������Һ֮��ķ�Ӧ������д���÷�Ӧ�Ļ�ѧ����ʽ ��

��1���������ѡ�������ᾧ�������ý��½ᾧ���������� ��
��2���ƴ�������У����й��˲�������Ҫ�IJ����������ձ���©���� ��
��3������ڷ�����Ӧ�Ļ�ѧ����ʽ�� ���������Ӧ������ ����þ�����һ��þ���Ͻ����������ɻ���ǵIJ��ϣ��ò��Ͼ��е���������
�� ���δ�һ�㣩��
��4������ܷ����ķ�ӦΪ�û���Ӧ�������ڽ���������Һ֮��ķ�Ӧ������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��1���Ȼ��Ƶ��ܽ�����¶�Ӱ���С ��2��������
��3��Mg(OH)2+2HCl = MgCl2+2H2O ���ֽⷴӦ �������ǿ�ȴ��ʴ
��4��2NaBr+Cl2= 2NaCl +Br2
��3��Mg(OH)2+2HCl = MgCl2+2H2O ���ֽⷴӦ �������ǿ�ȴ��ʴ
��4��2NaBr+Cl2= 2NaCl +Br2
����������Ȼ��Ƶ��ܽ�����¶�Ӱ��仯��������Ӧ��ȡ�����ܼ����ᾧ�����˲�������Ҫ�IJ����������ձ���©���Ͳ���������Ӧ��Ϊ������þ��ϡ���ᷴӦ�����Է�Ӧ����ʽΪMg(OH)2+2HCl = MgCl2+2H2O��������Ӧ����Ϊ���ֻ����ﻥ�ཻ���ɷ������������ֻ�����ķ�Ӧ�����ֽⷴӦ���Ͻ���ŵ㣬Ӳ�ȡ�ǿ��������ʴ������ǿ������ܷ����ķ�ӦΪ�û���Ӧ�������ڽ���������Һ֮��ķ�Ӧ�������Ը÷�Ӧ�Ļ�ѧ����ʽ2NaBr+Cl2= 2NaCl +Br2��

��ϰ��ϵ�д�
�����Ŀ
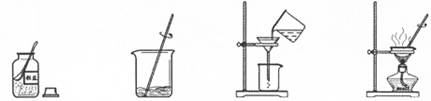

 Fe(OH)3��+3NaCl��x���ʾ��������������Һ������������ѡ����y���ʾ�ĺ���������ͼ��仯����һ����
Fe(OH)3��+3NaCl��x���ʾ��������������Һ������������ѡ����y���ʾ�ĺ���������ͼ��仯����һ���� 