��Ŀ����
��7�֣�������������������̼��һ����̼�dz��л�ѧ�еij������ʣ���ͼ�����ǵ�һЩ����Ӧ�á�

��������:
��1������������������˹�����������ǣ����������ƻ�ѧʽ����ͬ�� ��
��2��������������̼�����ϵ������� �����з����Ļ�ѧ����ʽΪ ��
��3����¯������һ����̼��ԭ�������Ļ�ѧ����ʽΪ ��
��4��������ȼ�ϵ������� ����ȼ�յĻ�ѧ����ʽΪ ��

| A��̼������ | B����¯���� | C���ɱ� | D��ȼ��ȼ�� |
��1������������������˹�����������ǣ����������ƻ�ѧʽ����ͬ�� ��
��2��������������̼�����ϵ������� �����з����Ļ�ѧ����ʽΪ ��
��3����¯������һ����̼��ԭ�������Ļ�ѧ����ʽΪ ��
��4��������ȼ�ϵ������� ����ȼ�յĻ�ѧ����ʽΪ ��
��1���ɱ�����CO2�� ����������������������������������������1��
��2��CO2 CO2 + H2O ="=" H2CO3 ������������������������������������2��
��3��3CO + Fe2O3 ="===" 2Fe + 3CO2 (���¡����ȶ�����)����������������2��
��4��CO��H2��ȱһ�����֣�
2CO + O2 2CO2 2H2 + O2
2CO2 2H2 + O2 2H2O ����������������2��
2H2O ����������������2��
��2��CO2 CO2 + H2O ="=" H2CO3 ������������������������������������2��
��3��3CO + Fe2O3 ="===" 2Fe + 3CO2 (���¡����ȶ�����)����������������2��
��4��CO��H2��ȱһ�����֣�
2CO + O2
 2CO2 2H2 + O2
2CO2 2H2 + O2 2H2O ����������������2��
2H2O ����������������2����1������������������˹�����������Ǹɱ�
��2��������������̼�����ϵ������Ƕ�����̼��
������̼��ˮ��Ӧ������̼�ᣬ��Ӧ�Ļ�ѧ����ʽΪ��CO2 + H2O ="=" H2CO3
��3����¯������һ����̼��ԭ�������Ļ�ѧ����ʽΪ��3CO + Fe2O3 2Fe + 3CO2
2Fe + 3CO2
��4��������ȼ�ϵ�������һ����̼��������
һ����̼������ȼ�յĻ�ѧ����ʽ�ֱ��ǣ�2CO + O2 2CO2 2H2 + O2
2CO2 2H2 + O2 2H2O
2H2O
��2��������������̼�����ϵ������Ƕ�����̼��
������̼��ˮ��Ӧ������̼�ᣬ��Ӧ�Ļ�ѧ����ʽΪ��CO2 + H2O ="=" H2CO3
��3����¯������һ����̼��ԭ�������Ļ�ѧ����ʽΪ��3CO + Fe2O3
 2Fe + 3CO2
2Fe + 3CO2��4��������ȼ�ϵ�������һ����̼��������
һ����̼������ȼ�յĻ�ѧ����ʽ�ֱ��ǣ�2CO + O2
 2CO2 2H2 + O2
2CO2 2H2 + O2 2H2O
2H2O 
��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ

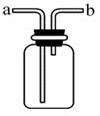
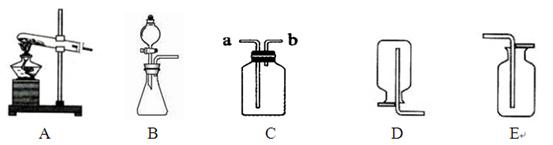
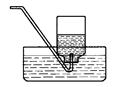
 ��д��ͼ�д�������������ƣ��� ��
��д��ͼ�д�������������ƣ��� ��