��Ŀ����
��ѧ�о���С��������к�ʵ�飮��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
��1��[����]�������ƹ�����¶�ڿ����в�������ˮ������������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ______��
[�������]����һ��������������Ʒ���ֱ��ʣ��������������������Ʒȫ�����ʣ�
��2��[��Ʒ���]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������������Թ��м�ˮ�ܽ⣬���������ij���ʯ��ˮ | ������ɫ���� | ����һ��ȷ |
| ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ | ��Һ��� |
��3��[ʵ��̽��]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������������Թ��м�ˮ�ܽ⣬����������______�� | ������ɫ���� | ����һ��ȷ |
| ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ | ��Һ��� |
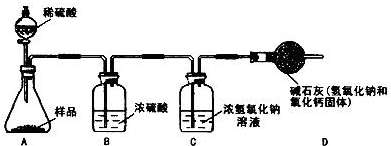
����Ϊ������Ҫ���������______������ĸ��ţ���
a��������Ʒ����������b����ʯ��ʵ��ǰ����������� c��Ũ���������
d��Ũ����������Һʵ��ǰ������������� e������ϡ���������
��5��[ʵ�鷴˼]�����������ݽ��м��㷢�ֽ����ʵ��ֵƫС��ԭ�������______��
�⣺��1���������ƺͿ����еĶ�����̼��Ӧ������̼���ƺ�ˮ���ʴ�Ϊ��2NaOH+CO2�TNa2CO3+H2O��
��2��ȡ������������Թ��м�ˮ�ܽ⣬������������ʯ��ˮ���а�ɫ�������ɣ�֤����Ʒ��һ����̼���ƣ���Ʒ���ʣ�̼������ʯ��ˮ��Ӧ�������������ƺ�̼��ƣ����ɵ���������Ҳ��ʹ��̪��죬����ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ����Һ���ɫ������֤����Һ�����������ƣ����Դ˷������������ʴ�Ϊ��̼������ʯ��ˮ��Ӧ��������������Ҳ��ʹ��Һ�еķ�̪��죻
��3��ȡ������������Թ��м�ˮ�ܽ⣬�μ�����CaCl2��Һ��������ɫ������֤����Ʒ��һ����̼���ƣ�ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ����Һ����ɫ��ɺ�ɫ��֤����Ʒ�����������ƣ����岿�ֱ��ʣ��������ȷ���ʴ�Ϊ���Ȼ�����Һ��
��4�������������ƹ�����Ʒ��̼���Ƶ������������㹫ʽ ��100%��Ҫ����̼���Ƶ�������������Ҫ֪��������Ʒ��������Ʒ��̼���Ƶ�������̼�����������ײ�����ɸ�������������Һʵ��ǰ���������������ɵĶ�����̼�������������̼�����������ʴ�Ϊ��ad��
��100%��Ҫ����̼���Ƶ�������������Ҫ֪��������Ʒ��������Ʒ��̼���Ƶ�������̼�����������ײ�����ɸ�������������Һʵ��ǰ���������������ɵĶ�����̼�������������̼�����������ʴ�Ϊ��ad��
��5�������������ݽ��м��㷢�ֽ����ʵ��ƫС���ټ��ϣ�4���ķ����ɵ�ԭ������ǣ�A��B�����ڻ���ʣ���CO2û����ȫ�������������գ���ʹ�����̼������������ƫС���ʴ�Ϊ��A��B�����ڻ���ʣ��Ķ�����̼û����ȫ��������������
��������1�����ݻ�ѧ����ʽ����д�������н��
��2������̼�������������Ƶ����ʽ��н��
��3������̼�������Ȼ��Ʒ�Ӧ������������Һ�ʼ��������
��4����5�������������ƹ�����Ʒ��̼���Ƶ������������㹫ʽ ��100%�����
��100%�����
���������⿼����ѧ����NaOH��Һ���տ����е�CO2����Na2CO3��Na2CO3��Һ�м��������Һ����CaCO3��ɫ�����ͷ�̪��Һ��ɫ����һЩ֪ʶ����Ŀͨ��ҩƷ�Ƿ���ʵ�̽��������ѧ����ʵ��̽���������ѶȽϴ�һ��Ҫ���������
��2��ȡ������������Թ��м�ˮ�ܽ⣬������������ʯ��ˮ���а�ɫ�������ɣ�֤����Ʒ��һ����̼���ƣ���Ʒ���ʣ�̼������ʯ��ˮ��Ӧ�������������ƺ�̼��ƣ����ɵ���������Ҳ��ʹ��̪��죬����ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ����Һ���ɫ������֤����Һ�����������ƣ����Դ˷������������ʴ�Ϊ��̼������ʯ��ˮ��Ӧ��������������Ҳ��ʹ��Һ�еķ�̪��죻
��3��ȡ������������Թ��м�ˮ�ܽ⣬�μ�����CaCl2��Һ��������ɫ������֤����Ʒ��һ����̼���ƣ�ȡ�ϲ���Һ������һֻ�Թ��У��μӷ�̪��Һ����Һ����ɫ��ɺ�ɫ��֤����Ʒ�����������ƣ����岿�ֱ��ʣ��������ȷ���ʴ�Ϊ���Ȼ�����Һ��
��4�������������ƹ�����Ʒ��̼���Ƶ������������㹫ʽ
 ��100%��Ҫ����̼���Ƶ�������������Ҫ֪��������Ʒ��������Ʒ��̼���Ƶ�������̼�����������ײ�����ɸ�������������Һʵ��ǰ���������������ɵĶ�����̼�������������̼�����������ʴ�Ϊ��ad��
��100%��Ҫ����̼���Ƶ�������������Ҫ֪��������Ʒ��������Ʒ��̼���Ƶ�������̼�����������ײ�����ɸ�������������Һʵ��ǰ���������������ɵĶ�����̼�������������̼�����������ʴ�Ϊ��ad����5�������������ݽ��м��㷢�ֽ����ʵ��ƫС���ټ��ϣ�4���ķ����ɵ�ԭ������ǣ�A��B�����ڻ���ʣ���CO2û����ȫ�������������գ���ʹ�����̼������������ƫС���ʴ�Ϊ��A��B�����ڻ���ʣ��Ķ�����̼û����ȫ��������������
��������1�����ݻ�ѧ����ʽ����д�������н��
��2������̼�������������Ƶ����ʽ��н��
��3������̼�������Ȼ��Ʒ�Ӧ������������Һ�ʼ��������
��4����5�������������ƹ�����Ʒ��̼���Ƶ������������㹫ʽ
 ��100%�����
��100%��������������⿼����ѧ����NaOH��Һ���տ����е�CO2����Na2CO3��Na2CO3��Һ�м��������Һ����CaCO3��ɫ�����ͷ�̪��Һ��ɫ����һЩ֪ʶ����Ŀͨ��ҩƷ�Ƿ���ʵ�̽��������ѧ����ʵ��̽���������ѶȽϴ�һ��Ҫ���������

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ
��ѧ�о���С��������к�ʵ�飮
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������______��
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���______������ţ�
A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ______��
[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
[����ʵ��]���������ǹ�ͬ��ɣ����ش����������⣺
[��չ]�о���С��ͬѧ�ڲ�����еμӵ��Լ�����ָʾ���⣬��������______�����
[��˼]NaOH����Ӧ______���森
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������______��
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���______������ţ�
A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ______��
[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
| ����Һ | CaCl2��Һ | Na2CO3��Һ | NaCl��Һ |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ��ȡ������Ʒ���Թ��У� ������ˮ��ȫ���ܽ⣬�� ��CaCl2��Һ����������ַ� Ӧ���ã� | ��ɫ���� | ��Ʒ��һ������______ |
| ����һ��ȡ����һ��ַ�Ӧ�� �������ϲ���Һ���Թ��У��� ����ɫ��̪��Һ���� | ______ | �������ʵ��ó� ����һ������ |
[��˼]NaOH����Ӧ______���森
��ѧ�о���С��������к�ʵ�飮
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������______��
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���______������ţ�
A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ______��
[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
[����ʵ��]���������ǹ�ͬ��ɣ����ش����������⣺
[��չ]�о���С��ͬѧ�ڲ�����еμӵ��Լ�����ָʾ���⣬��������______�����
[��˼]NaOH����Ӧ______���森
��ͬѧ������ʦָ���£����������ƹ�������100g������������Ϊ10%������������Һ��
��1���ܽ�ʱ��Ҫ�õ�����������������______��
��2�����в����ᵼ�����Ƶ�����������Һ���ʵ���������ƫ�͵���______������ţ�
A���������ƹ����к��в�����ˮ������
B����ˮϴ�����ȼ��δ�����ֱ����������
C������Ͳ��ȡˮ�����ʱ���ø��ӵķ�������
��С���ͬѧ��ϡ������������Ƶ�����������Һ�����к�ʵ��ʱ�����������ݲ�����С��ͬѧ�������ɣ�Ҫ����ƿ���ʵ��������ƹ������̽����
[����]�������ƹ�����¶�ڿ����в������գ�������������ж�����̼��Ӧ�����ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ______��
[�������]����һ��������������Ʒ���ֱ��ʣ�
�������������������Ʒȫ�����ʣ�
[��������]����֪��Ӧ��CaCl2+Na2CO3�T2NaCl+CaCO3��
�ڲ���������Һ��pH���±���
| ����Һ | CaCl2��Һ | Na2CO3��Һ | NaCl��Һ |
| pH | ����7 | ����7 | ����7 |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ����һ��ȡ������Ʒ���Թ��У� ������ˮ��ȫ���ܽ⣬�� ��CaCl2��Һ����������ַ� Ӧ���ã� | ��ɫ���� | ��Ʒ��һ������______ |
| ����һ��ȡ����һ��ַ�Ӧ�� �������ϲ���Һ���Թ��У��� ����ɫ��̪��Һ���� | ______ | �������ʵ��ó� ����һ������ |
[��˼]NaOH����Ӧ______���森
