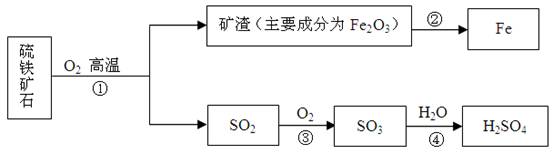
��Ŀ����
��ҵ����������ʯ����Ҫ�ɷ�FeS2���ڷ���¯�б��գ�����һϵ�з�Ӧ���õ����ᣮ���պ�Ŀ���������������ת���������£����������Ͳ�������ȥ����

��ش��������⣺
��1����ͼ���ֵĺ���Ԫ�ص������У��������������______���ѧʽ����
��2��FeS2�е���Ԫ����+2�ۣ���Ԫ�صĻ��ϼ�Ϊ______��
��3��д���ڴ������Ļ�ѧ����ʽ______������2000t������30%�Ŀ��������������Ͽɵõ�����98%����������Ϊ______ t��
��4����ú̿ȼ�չ�����Ҳ�����SO2����ҵ�Ͻ�úȼ�ղ���������ͨ������������ʯ��ˮ��ϴ��������������ã�ʹ֮��Ӧ��������ƺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ______��
�⣺��1��ͼ�г��ֵĺ���Ԫ�ص������У�SO2��SO3��������Ԫ����ɵ�����һ������Ԫ�صĻ���������������H2SO4�����⡢��������Ԫ����ɵĻ���������������
��2������Ԫ����+2������Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬��֪FeS2����Ԫ�صĻ��ϼۣ���+2��+2x=0����x=-1��
��3����ҵ���������û�ԭ��һ����̼���������ڸ����·�Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3 2Fe+3CO2��
2Fe+3CO2��
�������Ͽ���������98%����������Ϊx��
Fe2O3+3CO 3CO2+2Fe
3CO2+2Fe
160 112
2000t����1-30%�� 98%x
 x=1000t
x=1000t
��4���������⣬SO2��ʯ��ˮ��������Ӧ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O��
�ʴ�Ϊ����1��SO2��SO3����2��-1����3��3CO+Fe2O3 2Fe+3CO2��1000����4��2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O��
2Fe+3CO2��1000����4��2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O��
��������1������������ĸ����������ֻ������Ԫ�����Ǻ�����Ԫ�صĻ�������з������
��2�������ڻ��������������ϼ۴�����Ϊ�㣬���FeS2�Ļ�ѧʽ���н���⣮
��3�����������ķ�Ӧԭ����֪����ϻ�ѧ����ʽ����д���������裬д��������Ӧ�Ļ�ѧ����ʽ���ɣ��ɿ��������������ʵ����������������������������������������Ļ�ѧ����ʽ������ʽ���������������������
��4���������⣬������SO2��ʯ��ˮ��ϴ��������������ã�ʹ֮��Ӧ��������ƺ�ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
�����������ѶȲ�����������������������û��ϼ۵�ԭ�����ָ��Ԫ�صĻ��ϼ۵ķ�������ѧ����ʽ����д���������������ʵĻ�ѧ����ʽ�ļ��������ȷ�����Ĺؼ���
��2������Ԫ����+2������Ԫ�صĻ��ϼ���x�������ڻ��������������ϼ۴�����Ϊ�㣬��֪FeS2����Ԫ�صĻ��ϼۣ���+2��+2x=0����x=-1��
��3����ҵ���������û�ԭ��һ����̼���������ڸ����·�Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3
 2Fe+3CO2��
2Fe+3CO2���������Ͽ���������98%����������Ϊx��
Fe2O3+3CO
 3CO2+2Fe
3CO2+2Fe160 112
2000t����1-30%�� 98%x
 x=1000t
x=1000t��4���������⣬SO2��ʯ��ˮ��������Ӧ��������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O��
�ʴ�Ϊ����1��SO2��SO3����2��-1����3��3CO+Fe2O3
 2Fe+3CO2��1000����4��2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O��
2Fe+3CO2��1000����4��2SO2 +2Ca��OH��2 +O2 �T2CaSO4 +2H2O����������1������������ĸ����������ֻ������Ԫ�����Ǻ�����Ԫ�صĻ�������з������
��2�������ڻ��������������ϼ۴�����Ϊ�㣬���FeS2�Ļ�ѧʽ���н���⣮
��3�����������ķ�Ӧԭ����֪����ϻ�ѧ����ʽ����д���������裬д��������Ӧ�Ļ�ѧ����ʽ���ɣ��ɿ��������������ʵ����������������������������������������Ļ�ѧ����ʽ������ʽ���������������������
��4���������⣬������SO2��ʯ��ˮ��ϴ��������������ã�ʹ֮��Ӧ��������ƺ�ˮ��д����Ӧ�Ļ�ѧ����ʽ���ɣ�
�����������ѶȲ�����������������������û��ϼ۵�ԭ�����ָ��Ԫ�صĻ��ϼ۵ķ�������ѧ����ʽ����д���������������ʵĻ�ѧ����ʽ�ļ��������ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
��ҵ����������ʯ����Ҫ�ɷ�FeS2���ڷ���¯�б��գ�����һϵ�з�Ӧ���õ����ᡣ���պ�Ŀ���������������ת���������£����������Ͳ�������ȥ����
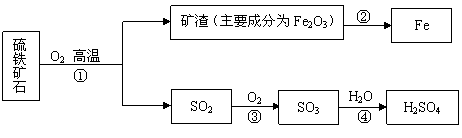 |
��![]() �ش��������⣺
�ش��������⣺
��1����ͼ���ֵĺ���Ԫ�ص������У�������������� ���ѧʽ����
��2��FeS2�е���Ԫ����+2�ۣ���Ԫ�صĻ��ϼ�Ϊ ��
��3��д���ڴ������Ļ�ѧ����ʽ ������2000 t������30%�Ŀ��������������Ͽɵõ�����98%����������Ϊ_________ t��
��4����ú̿ȼ�չ�����Ҳ�����SO2����ҵ�Ͻ�úȼ�ղ���������ͨ������������ʯ��ˮ��ϴ��������������ã�ʹ֮��Ӧ��������ƺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ ��