��Ŀ����
����
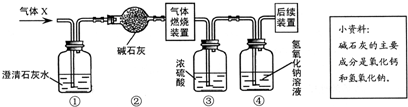
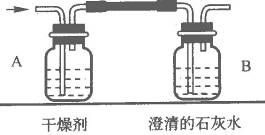
x���ܺ���H2��CO��CH4�е�һ�ֻ��֣���xͨ����������ȫȼ�պ��ٽ�����������ͨ����A��B�����ӵ�װ����(��ͼ��ʾ)������ʵ����������ȷ���������ɣ��Իش�
(1)��װ��A���������ӣ�B���������䣬��xΪ________________��ȼ�յĻ�ѧ����ʽΪ________________��
(2)��װ��A���������䣬B���������ӣ���xΪ________________��Bƿ�з�Ӧ�Ļ�ѧ����ʽΪ________________��
(3)��װ��A��B�����������ӣ���x����Ϊ________________________��
�𰸣�
������
������
|
���� (1)H2,2H2��O2���� (2)CO,CO2��Ca(OH)2���� (3)CH4��CO��H2��CH4��H2��CH4��CO��H2��CO��CH4���� H2��CO��CH4����������������ȼ�յIJ���ֱ�ΪH2O��CO2��H2O��CO2��ͨ����֤����ȼ�յIJ�������������ǣ��������������ˮ������ʯ��ˮ��������CO2���أ�ͨ��A��Bװ�����أ��ж�����ȼ�ղ�����������ж��Ǻ������壮��Ҫע�⣬ȼ�պ�ͬʱ��H2O��CO2���������������CH4��Ҳ����Ϊ���������е����ֻ�������ɵĻ�����壮 |

��ϰ��ϵ�д�
�����Ŀ