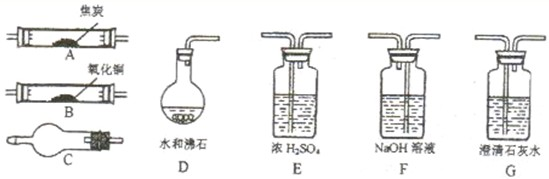
��Ŀ����
ˮ����ͨ�����ȵĽ�̿�������������Ҫ�ɷ���CO��H2������CO2��ˮ�����ȣ�������ͼ���ṩ��������ѡ���Ҫ���Լ������һ��ʵ�飬֤�����������������CO��H2��������װ�ú͵��ܵ���ͼ����ȥ��
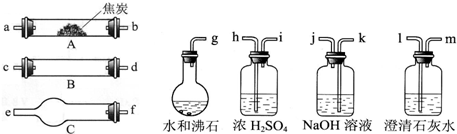
�ش��������⣺
��1������B��C���������Լ������ƣ���ѧʽ���ֱ��ǣ�
��2���������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��g-a b-
��3����֤����������к���H2��ʵ�������ǣ�

�ش��������⣺
��1������B��C���������Լ������ƣ���ѧʽ���ֱ��ǣ�
����ͭ��CuO������ˮ����ͭ��CuSO4��
����ͭ��CuO������ˮ����ͭ��CuSO4��
����2���������������Ӹ�����������ĸ��ʾ�ӿڵ�����˳��g-a b-
kj-hi-cd����dc��-fe-lm
kj-hi-cd����dc��-fe-lm
����3����֤����������к���H2��ʵ�������ǣ�
B�к�ɫ��CuO��ɺ�ɫ��ĩ��C����ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ
B�к�ɫ��CuO��ɺ�ɫ��ĩ��C����ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ
����������1����������ͭ��CO��H2��Ӧ�Լ�ˮʹ��ˮ����ͭ�������з�����
��2������ʵ�����ͼ��������������Ⱥ�˳��
��3�����������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ������з�����
��2������ʵ�����ͼ��������������Ⱥ�˳��
��3�����������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ������з�����
����⣺��1���������������CO��H2��������ͭ������ˮ������ˮ����ͭ���������ͭ��CuO������ˮ����ͭ��CuSO4��
��2��Ϊ����֤������к���CO��H2��ʵ�������������Ⱥ�˳��Ϊ����������̼������ˮ�֡���ԭװ�á�����ˮ�֡����������̼������������������ԭ���ǣ������̳��������g-a b��-k j-h i-c d����d c��-f e-l m
��3��������������ڣ������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ���壬���B�к�ɫ��CuO��ɺ�ɫ��ĩ��C����ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ��
��2��Ϊ����֤������к���CO��H2��ʵ�������������Ⱥ�˳��Ϊ����������̼������ˮ�֡���ԭװ�á�����ˮ�֡����������̼������������������ԭ���ǣ������̳��������g-a b��-k j-h i-c d����d c��-f e-l m
��3��������������ڣ������ͺ�ɫ������ͭ��Ӧ���ɺ�ɫ��ͭ��ˮ��ˮ�Ͱ�ɫ����ˮ����ͭ��Ӧ������ɫ������ͭ���壬���B�к�ɫ��CuO��ɺ�ɫ��ĩ��C����ˮ����ͭ�ɰ�ɫ��Ϊ��ɫ��
�������ش���Ҫ֪��������ʱҪ��ˮ�ŵ�����ȥ�������֤ˮ�Ĵ���ʱҪ����֤ˮ���ڵ���ˮ����ͭ������ǰ�森

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ