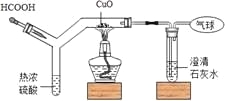
��Ŀ����
����Ŀ��ȡ200gһ������������CuSO4��Һ������εμ�100gBa��OH��2��Һ��b�㣬������εμ�ϡHCl��Һ�����������������м�����Һ�����������������ϵ��ͼ��ʾ����ش�������⣺
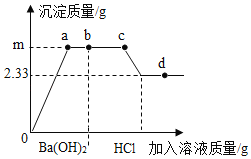
��1��oa ����Һ�ĵ����Խ���_____�����ǿ�������������������䡱����
��2����d��ʱ��Һ��������������_____��
��3��bc�η�����Ӧ�Ļ�ѧ����ʽ_____��
��4��ͨ������ȷ��m��ֵ_____��
���𰸡����� BaCl2��CuCl2��HCl Ba��OH��2+2HCl��BaCl2+2H2O 3.31g
��������
��1��ͨ������ͼ���֪��o��a����������������ͭ��Ӧ�������ᱵ������������ͭ��������Һ������������
��2��b�㿪ʼʣ������������Լ����ɵ�������ͭ��ʼ�����ᷴӦ������ͼ����˼�������Ⱥ�����������Ӧ��ȫ��ź�������ͭ��Ӧ��d��ʱ������������Դ�ʱ������Ϊ�Ȼ������Ȼ�ͭ���Ȼ��⣬��Ӧ�Ļ�ѧʽΪ��BaCl2��CuCl2��HCl��
��3��bc�η����ķ�Ӧ���������������ᷴӦ�����Ȼ�����ˮ����ѧ����ʽΪ��Ba��OH��2+2HCl��BaCl2+2H2O��
��4���⣺��ͼ���е����ݿ�֪�������ᱵ����������Ϊ2.33g��
������������ͭ����������Ϊx���μӷ�Ӧ����������������Ϊy��
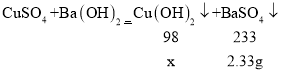
![]()
��ã�x��0.98g������m��0.98g+2.33g��3.31g��

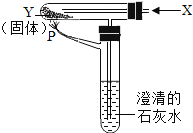
����Ŀ������������۲졢ѧϰ����һ��ʵ�顣ȡ�Ķ���ʯ����ҺȾ����ɫ�ĸ����ֽ�����ֱ���ͼ����ʵ�顣
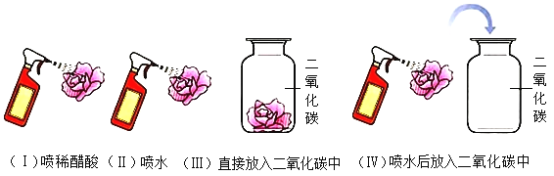
��1�������ͼʾ����˼�����ش��±��е����⣺
��I�� | ������ | ������ | ������ | |
���� | ��ɫֽ����� | ��ɫֽ������ɫ | ��ɫֽ������ɫ | _____�� |
���� | ��ʵ�飨������֤��_____����ʵ�飨������֤��_____�� |
��2��ʵ�飨����������ֽ���þƾ���С�ļ��ȹ�����ֽ���ֱ��ϣ�˵��̼��_____��д���÷�Ӧ�Ļ�ѧ����ʽΪ_____��