��Ŀ����
��1��С���ڹ���Ĵ�װ��Ƭ�з�����һ��С����������С��ɷ֣�CaO��Fe�����ã������������������棺��ֹʳ�á���������˺��С������������Ĺ����ĩ���������غ�ɫ�ģ�������ǻҰ�ɫ�ģ���������������Ϊ�غ�ɫ��ĩ��Fe2O3���Ұ�ɫ��ĩӦ�ú���CaCO3��Fe��Ϊ��֤�Լ����жϣ�С���������ʵ��̽��������һͬ���룮
ʵ��Ŀ�ģ�֤ʵ�Ұ�ɫ��ĩ�к���CaCO3��Fe��
�������ϣ����������л�ԭ�ԣ��ܶ�ȡijЩ�����������е�����ʹ������ԭ���ڰ�ɫ����ˮ����ͭ��ˮ�����ɫ��
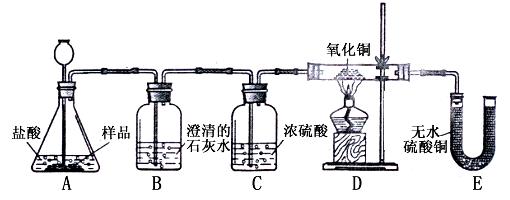
ʵ����ƣ�װ����ͼ��ʾ��

ʵ��Ԥ���������
����Ҫ֤ʵ��Ʒ�к���CaCO3��B�й۲쵽��ʵ��������
����Ҫ֤ʵ��Ʒ�к���Fe��D��Ӧ�۲쵽��ʵ��������
�ۿ���С���ķ������ۣ���ͬѧ��ΪС���ڻҰ�ɫ��ĩ�л�Ӧ���б�����ʣ�����д������һ�����ʵĻ�ѧʽ
��С����Ϊ�غ�ɫ��ĩFe2O3Ҳ�������ᷴӦ������д����Ӧ�Ļ�ѧ����ʽ
��2����ͭ��ͭ��п�Ͻ�������������������������������Ʒ��Ϊ�ⶨij��ͭ��ͭ��������������ȡ20g����Ʒ���飬���뵽100gϡ�����У�ǡ����ȫ��Ӧ����Ӧ�����ձ���ʣ�����������Ϊ119.6�ˣ�
�ٷ�Ӧ����������������
�ڻ�ͭ��Ʒ��ͭ������������
�ۼ���ϡ���������ʵ�����������
ʵ��Ŀ�ģ�֤ʵ�Ұ�ɫ��ĩ�к���CaCO3��Fe��
�������ϣ����������л�ԭ�ԣ��ܶ�ȡijЩ�����������е�����ʹ������ԭ���ڰ�ɫ����ˮ����ͭ��ˮ�����ɫ��
ʵ����ƣ�װ����ͼ��ʾ��

ʵ��Ԥ���������
����Ҫ֤ʵ��Ʒ�к���CaCO3��B�й۲쵽��ʵ��������
����ʯ��ˮ�����
����ʯ��ˮ�����
��B�з�����Ӧ�Ļ�ѧ����ʽΪCa��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
������Ҫ֤ʵ��Ʒ�к���Fe��D��Ӧ�۲쵽��ʵ��������
��ɫ�����ɺ�ɫ
��ɫ�����ɺ�ɫ
��E��Ӧ�۲쵽��ʵ����������ɫ��������ɫ
��ɫ��������ɫ
���ۿ���С���ķ������ۣ���ͬѧ��ΪС���ڻҰ�ɫ��ĩ�л�Ӧ���б�����ʣ�����д������һ�����ʵĻ�ѧʽ
CaO��Ca��OH��2
CaO��Ca��OH��2
����С����Ϊ�غ�ɫ��ĩFe2O3Ҳ�������ᷴӦ������д����Ӧ�Ļ�ѧ����ʽ
Fe2O3+6HCl=2FeCl3+3H2O
Fe2O3+6HCl=2FeCl3+3H2O
����2����ͭ��ͭ��п�Ͻ�������������������������������Ʒ��Ϊ�ⶨij��ͭ��ͭ��������������ȡ20g����Ʒ���飬���뵽100gϡ�����У�ǡ����ȫ��Ӧ����Ӧ�����ձ���ʣ�����������Ϊ119.6�ˣ�
�ٷ�Ӧ����������������
0.4g
0.4g
���ڻ�ͭ��Ʒ��ͭ������������
35%
35%
���ۼ���ϡ���������ʵ�����������
��������1��������Ʒ�к���CaCO3�����������ᷴӦ���������壻�����������������ʯ��ˮ��Ӧ��
������Ʒ�к���Fe���������ᷴӦ�����ܻ�ԭ����ͭ����������ԭ����ͭʱ��ˮ���ɣ�
����Ʒ�гɷֿ���δ��ȫ���ʣ����ܺ��������ƣ������������ƺ�ˮ��Ӧ�����������Ƶ����ʽ��
��2���ٸ���ͭ��п�����ʿ�֪������ϡ����ʱ��ֻ��п�����ᷴӦ����������п�����������������غ㶨�ɿ�֪�����ٵ�������������������������
�ڸ���п�����ᷴӦ�ķ���ʽ���������������������п���������������ͭ��������ͭ������������
�۸��ݷ���ʽ�͡�������������=
��100%�����㼴�ɣ�
������Ʒ�к���Fe���������ᷴӦ�����ܻ�ԭ����ͭ����������ԭ����ͭʱ��ˮ���ɣ�
����Ʒ�гɷֿ���δ��ȫ���ʣ����ܺ��������ƣ������������ƺ�ˮ��Ӧ�����������Ƶ����ʽ��
��2���ٸ���ͭ��п�����ʿ�֪������ϡ����ʱ��ֻ��п�����ᷴӦ����������п�����������������غ㶨�ɿ�֪�����ٵ�������������������������
�ڸ���п�����ᷴӦ�ķ���ʽ���������������������п���������������ͭ��������ͭ������������
�۸��ݷ���ʽ�͡�������������=
| ���ʵ����� |
| ��Һ������ |
����⣺��1��������Ʒ�к���CaCO3��CaCO3�������ᷴӦ���ɶ�����̼���壬�۲쵽��ʵ�����������ɵ����������̼����B�������е����еij���ʯ��ˮ��Ӧ���ɳ�������Ӧ����ʽ��CO2+Ca��OH��2=CaCO3��+H2O��
�ʴ�Ϊ�������ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O��
������Ʒ�к���Fe��Fe�����ᷴӦ������������������Dװ�ú�ԭ����ͭ������ͭ��ˮ����Ӧ�к�ɫ��ĩ��Ϊ��ɫ��ˮ��������Eװ�ã����Կ���E�е���ˮ����ͭ������
�ʴ�Ϊ����ɫ�����ɺ�ɫ�� ��ɫ��������ɫ��
����ΪС��������гɷ֣�CaO��Fe�����е�CaO�����в���δ���ʣ����ܻ����տ����е�ˮ�֣�����Ca��OH��2�����߶��ǻҰ�ɫ���壬���ԻҰ�ɫ��ĩ�л�Ӧ����CaO��Ca��OH��2��
�ʴ�Ϊ��CaO��Ca��OH��2��
��2���ٸ��������غ㶨�ɿ�֪�����ɵ�����������Ϊ��100g+20g-119.6g=0.4g
�ʴ�Ϊ��0.4g
������Ʒ��п��������X����ϡ���������ʵ�����ΪY
Zn+H2SO4=ZnSO4+H2��
65 98 2
X Y 0.4g
=
=
��ã�X=13g Y=19.6g
������Ʒ��ͭ������Ϊ��20g-13g=7g
��Ʒ��ͭ������������
��100%=35%
�ʴ�Ϊ��35%��
��ϡ�����е����ʵ���������=
��100%=19.6%
��ϡ���������ʵ���������Ϊ19.6%
�ʴ�Ϊ�������ʯ��ˮ����ǣ�CO2+Ca��OH��2=CaCO3��+H2O��
������Ʒ�к���Fe��Fe�����ᷴӦ������������������Dװ�ú�ԭ����ͭ������ͭ��ˮ����Ӧ�к�ɫ��ĩ��Ϊ��ɫ��ˮ��������Eװ�ã����Կ���E�е���ˮ����ͭ������
�ʴ�Ϊ����ɫ�����ɺ�ɫ�� ��ɫ��������ɫ��
����ΪС��������гɷ֣�CaO��Fe�����е�CaO�����в���δ���ʣ����ܻ����տ����е�ˮ�֣�����Ca��OH��2�����߶��ǻҰ�ɫ���壬���ԻҰ�ɫ��ĩ�л�Ӧ����CaO��Ca��OH��2��
�ʴ�Ϊ��CaO��Ca��OH��2��
��2���ٸ��������غ㶨�ɿ�֪�����ɵ�����������Ϊ��100g+20g-119.6g=0.4g
�ʴ�Ϊ��0.4g
������Ʒ��п��������X����ϡ���������ʵ�����ΪY
Zn+H2SO4=ZnSO4+H2��
65 98 2
X Y 0.4g
| 65 |
| X |
| 2 |
| 0.4g |
| 98 |
| Y |
| 2 |
| 0.4g |
��ã�X=13g Y=19.6g
������Ʒ��ͭ������Ϊ��20g-13g=7g
��Ʒ��ͭ������������
| 7g |
| 20g |
�ʴ�Ϊ��35%��
��ϡ�����е����ʵ���������=
| 19.6g |
| 100g |
��ϡ���������ʵ���������Ϊ19.6%
���������⿼�������ʵĻ�ѧ���ʼ���Ӧʱ��������ݻ�ѧ����ʽ�ļ��㣬��������������ʵĻ�ѧ���ʡ�����ʽ���㣬�ٽ�������龳���ѽ��

��ϰ��ϵ�д�
�����Ŀ