��Ŀ����
��3�֣���ʢ��22.3 g Na2CO3��NaCl����������ձ��м���216.1 gϡ����ǡ�÷�Ӧ,��Ӧ�����þ�����������ձ���ͬҩƷ������(m)�뷴Ӧʱ��(t)�Ĺ�ϵ����ͼ��ʾ���ձ���ͬҩƷ����ʼ����Ϊ300 g��

�ش��������⣺
(1)������������ϡ����ǡ����ȫ��Ӧʱ������ʱ��ԼΪ S��
(2)��ȫ��Ӧ����������̼�������� g��
(3)��Ӧ�õ������µIJ�������Һ��������Һ�����ʵ���������Ϊ���٣�

�ش��������⣺
(1)������������ϡ����ǡ����ȫ��Ӧʱ������ʱ��ԼΪ S��
(2)��ȫ��Ӧ����������̼�������� g��
(3)��Ӧ�õ������µIJ�������Һ��������Һ�����ʵ���������Ϊ���٣�
��1��20��2��4.4��3��10%
�����������1�������е�ͼʾ��֪��������������ϡ���ᷴӦ20Sʱ���������������ٸı䣬��ʱǡ�÷�Ӧ��ȫ��
��2����ȫ��Ӧ����������̼��������=300.0-295.6=4.4g��
��3���⣺�������е�̼���Ƶ�����ΪX����Ӧ�������Ȼ��Ƶ�����ΪY��
Na2CO3+2HCl=2NaCl+
 +
+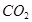 ��
��106 117 44
X Y 4.4g
106��44=X��4.4g
X=10.6g
117��44=Y��4.4g
Y=11.7g
��Ӧ��������Һ�����ʵ���������Ϊ
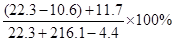 =10%��
=10%�����������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢��
��Һ��������������=
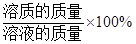

��ϰ��ϵ�д�
�����Ŀ

 Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ�
Si+ 4HCl�������Ҫ���56g�裨Si����������Ҫ�������ٿˣ�
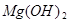 +2HCl=
+2HCl= +H2O��
+H2O��