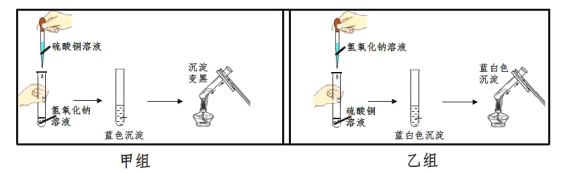
��Ŀ����
����Ŀ����2016��ɽ��ʡ�Ͳ��С��Ͻ������ܶ࣬��;�dz��㷺����ͭ��ͭ��п�ĺϽ�������������������͵���������ȣ���ѧ��ȤС���ͬѧ���ⶨʵ������ijͭ��Ʒ��ͭ�����������������ǻ�ͭ�е��������ʣ�������������ǵ�̽�����̡�
����10g��ĩ״��ͭ��Ʒ�����ձ��У���ȡ45mLϡ��������μӵ����У�ÿ�γ�ַ�Ӧ�ⶨ����������������ʵ�����������
��һ�� | �ڶ��� | ������ | |
����ϡ����������ml�� | 15 | 15 | 15 |
����������������g�� | 0.04 | m | 0.02 |
����
��1��m����ֵ ��
��2���˻�ͭ��Ʒ��ͭ�����������Ƕ��٣���д��������̣�
���𰸡���1��0.04��2��67.5%
�������������������1�����ݱ�����Է��֣���һ�μ���15mL��������0.04g�����������μ���15mL���ỹ�����ɲ���ֻ����0.02g������˵��ֱ�������η�Ӧ�Ž���������������ʣ�ࣻͬʱ˵���ڶ��μ���15mL����ʱ��ȫ��Ӧ�����������������ɰ��յ�һ�ε�������ϵ��15ml��Ӧ0.04g�����ƣ����ó�mΪ0.04g��
��2�����ݱ����֪������������������Ϊ0.04g+0.04g+0.02g=0.1g������Ҫп������Ϊx������
Zn+2HCl=ZnCl2+H2��
65 2
x 0.1g
![]()
x=3.25g
���Դ˻�ͭ��Ʒ��ͭ����������Ϊ��![]() 100%=67.5%��
100%=67.5%��

 ��У����ϵ�д�
��У����ϵ�д�