��Ŀ����
��2007?�ൺ��Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ� ��Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ���]
��1������һ����һ������Ʒ�м����������ʯ��ˮ�����ݷ�Ӧ����̼��Ƶ������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
��2������������һ������Ʒ�м���������ϡ���ᣬ���ݷ�Ӧ���ɶ�����̼�������������̼���Ƶ��������ټ�����Ʒ��̼���Ƶ�����������
[����ʵ��]
����ͬѧ����ȡ12.00g��Ʒ����ˮ�����Һ������Һ�м�������ij���ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00g��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.40g������̼��
[�������]������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ���������������������ȷ��0.1% ��
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ����������______����һ�־������ʣ�����Һ����Ʒ��Ӧ��ͨ���ⶨ������ʵ������������йؼ��㼴�ɣ�
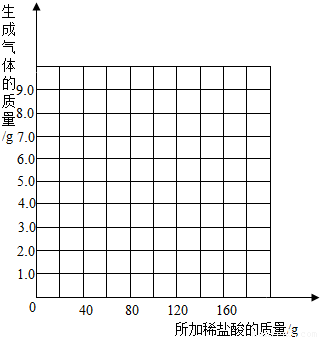
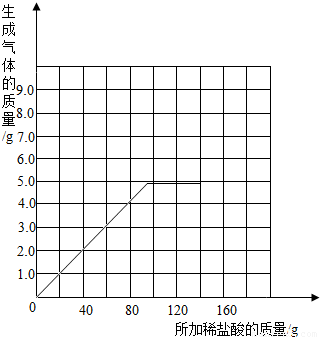
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ18.45g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
| ����ϡ������� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| �ձ�����������������/g | 78.90 | 97.80 | 116.70 | 135.60 | 155.05 | 175.05 | 195.05 |
���𰸡���������1�����������֪����ͬѧ���õ��Ƿ���һ��������̼����ת���ɳ������ٸ��ݳ����������̼�����������������̼���Ƶ�������������ô��̼����ת��Ϊ������Ҫ�����ε������Լ�̼���ε��ܽ�ȱ���ѡ���Լ���
��2�������������Ϳ��Եõ�ÿ�μ�ϡ��������õĶ�����̼��������Ȼ���������������ֽ�ϰ�������������������ϡ���������Ĺ�ϵ�����Ƴ������ɣ�
����⣺[�������]�����ѡ����ͬѧ��ʵ�������У���μӷ�Ӧ��̼���Ƶ�����Ϊx
Na2CO3+Ca��OH��2�T2NaOH+CaCO3��
106 100
x 10.00g
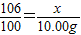 x=10.6g
x=10.6g
������Ʒ��̼���Ƶ���������Ϊ��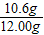 ×100%=88.3%
×100%=88.3%
�����ѡ������ͬѧ�Ľ�����У���μӷ�Ӧ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.40g
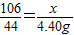 x=10.6g
x=10.6g
������Ʒ��̼���Ƶ���������Ϊ��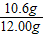 ×100%=88.3%
×100%=88.3%
[������˼]��1��̼���Ƴ��˿��Ժ�ʯ��ˮ��Ӧ���ɳ����⣬�����Ժ��������ʷ�Ӧ���ɳ�������Ϊ̼��ơ�̼�ᱵ�ȶ��dz���������̼���ƿ��Ժ��Ȼ��ơ��Ȼ������η�Ӧ��
��2���ɱ��������ݿ�֪ǰ4��ʵ��ÿ�μ���20gϡ������ձ�����Һ��������ǰһ��������18.9g����ǰ4��ʵ��ÿ�����ɶ�����̼����1.1g����5�μ���20gϡ��������Ķ�����̼����ֻ��0.55g��˵����ʱ̼��������ȫ��Ӧ����ϡ��������80g��100g֮��ʱ�������Ķ�����̼������=1.1g×4+0.55g=4.95g�����Ժ������ټӶ���ϡ���ᣬ������̼�������ִ�ֵ���䣮�����Ϸ�������������ֽ�ϻ����������������������ϡ����������ϵ������ͼΪ��
������������һ���ۺ��Ե���Ŀ�����м��и��ݻ�ѧ����ʽ�ļ��㣬���и��ݱ����е����ݻ������ߣ������ѶȱȽϴ����Ŀ��
��2�������������Ϳ��Եõ�ÿ�μ�ϡ��������õĶ�����̼��������Ȼ���������������ֽ�ϰ�������������������ϡ���������Ĺ�ϵ�����Ƴ������ɣ�
����⣺[�������]�����ѡ����ͬѧ��ʵ�������У���μӷ�Ӧ��̼���Ƶ�����Ϊx
Na2CO3+Ca��OH��2�T2NaOH+CaCO3��
106 100
x 10.00g
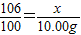 x=10.6g
x=10.6g������Ʒ��̼���Ƶ���������Ϊ��
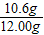 ×100%=88.3%
×100%=88.3%�����ѡ������ͬѧ�Ľ�����У���μӷ�Ӧ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.40g
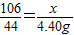 x=10.6g
x=10.6g������Ʒ��̼���Ƶ���������Ϊ��
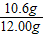 ×100%=88.3%
×100%=88.3%[������˼]��1��̼���Ƴ��˿��Ժ�ʯ��ˮ��Ӧ���ɳ����⣬�����Ժ��������ʷ�Ӧ���ɳ�������Ϊ̼��ơ�̼�ᱵ�ȶ��dz���������̼���ƿ��Ժ��Ȼ��ơ��Ȼ������η�Ӧ��
��2���ɱ��������ݿ�֪ǰ4��ʵ��ÿ�μ���20gϡ������ձ�����Һ��������ǰһ��������18.9g����ǰ4��ʵ��ÿ�����ɶ�����̼����1.1g����5�μ���20gϡ��������Ķ�����̼����ֻ��0.55g��˵����ʱ̼��������ȫ��Ӧ����ϡ��������80g��100g֮��ʱ�������Ķ�����̼������=1.1g×4+0.55g=4.95g�����Ժ������ټӶ���ϡ���ᣬ������̼�������ִ�ֵ���䣮�����Ϸ�������������ֽ�ϻ����������������������ϡ����������ϵ������ͼΪ��

������������һ���ۺ��Ե���Ŀ�����м��и��ݻ�ѧ����ʽ�ļ��㣬���и��ݱ����е����ݻ������ߣ������ѶȱȽϴ����Ŀ��

��ϰ��ϵ�д�
�����Ŀ
 ��2007?�ൺ����ˮɹ�κ�õ��ľ����Ǵ��Σ�ʣ���Һ���Ϊ��±����±�г��Ȼ�������ж��ֳɷ֣���ʳƷ�������ȷ�������ҪӦ�ã��������֪��Ϣ�ش��������⣮
��2007?�ൺ����ˮɹ�κ�õ��ľ����Ǵ��Σ�ʣ���Һ���Ϊ��±����±�г��Ȼ�������ж��ֳɷ֣���ʳƷ�������ȷ�������ҪӦ�ã��������֪��Ϣ�ش��������⣮