��Ŀ����
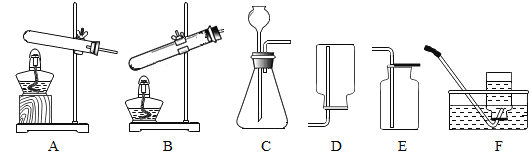
����Ŀ�������Ļ������þ�����Ҫ�����壬ij�Ͼɽ����к�����������Ni�������⣨ֻ�����������������������ɷ֣���ͭ��Ϊ����ijЩ��������ȡij�־��壬���������ʵ�飺��֪�� ![]()
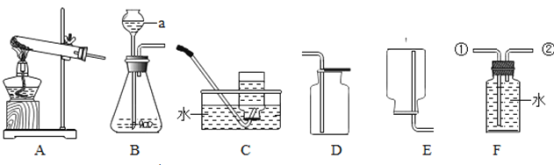
�������bΪ����������ش��������⣻
��1����������������ʵ�����A������Ϊ_____��
��2��ͨ������ʵ�����̣���֪����ͭ�����Ļ˳��Ϊ_____��
��3�������Ͼɽ����е����������Ҳ�ܷ�����Ӧ��д���˷�Ӧ�Ļ�ѧ����ʽ��_____��
���𰸡����� Fe>Ni>Cu ![]()
��������
��1����������������ʵ�����A�������Һ����룬���ڹ��˲�����������ˣ�
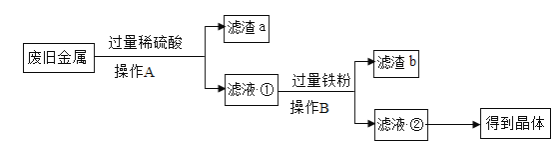
��2������A�У�����������ϡ���ᷴӦ��ͭ����ϡ���ᷴӦ��˵��ͭ����ã�����B�У�������������������û���Һ�еĽ����������յõ�����������Һ��˵�����Ļ�Դ����������Fe>Ni>Cu��
��3���������Ҫ�ɷ����������������������ᷴӦ������������ˮ����Ӧ�Ļ�ѧ����ʽ��Ϊ��![]() �����
�����![]() ��
��

����Ŀ�����ɺͱȽ���ѧϰ��ѧ����Ҫ�������Դ�ͬһ�����⣬�Ӳ�ͬ�Ƕ����ǿ����ҵ���ͬ�Ĺ��ɣ��Ӷ��ó���ͬ�Ĵ𰸡��밴��Ҫ��ʾ����д�±������ڲ�ͬ������
���� | ���ڲ�ͬ������ | ���� |
KCl��K2SO4��K2CO3��BaCO3 | KCl | KCl�в�����Ԫ�أ����������ж�����Ԫ�� |
_________ | _________ |
��2��������һ����ɫ���д̼�����ζ��Һ�壬�н�ǿ�ĸ�ʴ�ԡ�С����֪�������Ƿ�������ԣ���������̽����
���������IJ��������������������������Ϊ������һ��ʵ������ܹ�������һ���룿����ͼ����ĸ��ʾ___________��
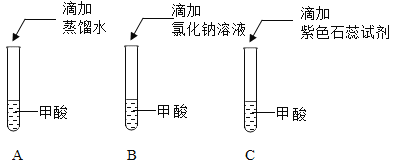
����С���IJ�������ȷ�ģ������ͻ�����������������̼���Ʒ�Ӧ����֪���ᣨHCOOH����ˮ���ӵ����ÿ��Խ����H+��HCOO-����д��������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��____________��