��Ŀ����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ�ú�ϰ���г���Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ��������벻����Ч����
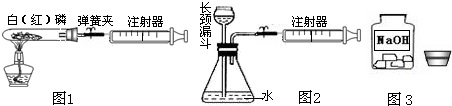
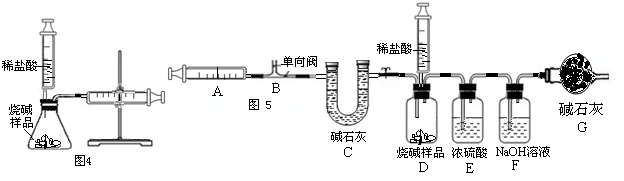
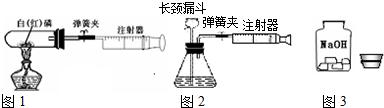
��1��ͼ1��50 mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���20 mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ�������������Ϊ����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�ԼmL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ�����װ�����������ã����ܹ۲쵽A��ע��������Һ��B��ƿ��Һ������C������©����Һ������D������©���¶˹ܿڲ�������
��3����ȤС���߽�ʵ���ҿ�����һ������г�ġ�����������ͼ3����
��I������˾��������뵽��ȡ�ø�ҩƷʱ�Լ�ƿƿ��Ӧ�������ϣ�ȡ�ú�Ӧ��������ƿ���ܷⱣ�棬������Ϊ��
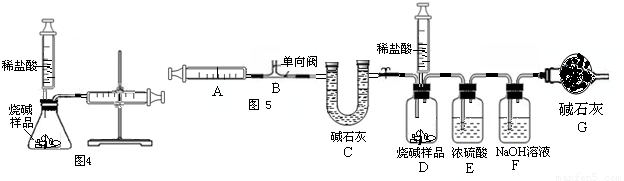
��II������ȤС��ͬѧΪ�˲ⶨij���ʵ��ռ���Ʒ��Na2CO3���������������������ͼ4��ʾ��װ�ã�ͨ����Ӧ���Ҳ�ע�������ռ���������������Na2CO3��������������֪��״̬��CO2���ܶȣ���ʹ��ע�����������Ϊ20mL����д����ƿ���������仯�Ļ�ѧ����ʽ����
С����Ϊ�÷�������ȷ���Na2CO3������������������Ϊ����С����Ϊ�÷�������Ʒ��ȡ�õ���Ҳ��Ҫһ���Ŀ��ƣ�������Ϊ��
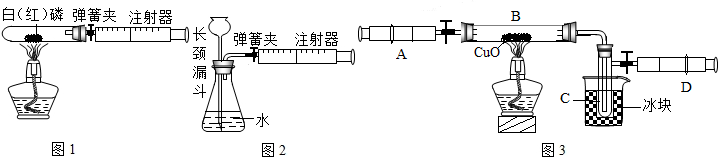
��III������ȤС��ͬѧ�ڷ�˼����ʵ����������ͼ5��װ�òⶨ���ʵ��ռ���Ʒ��Na2CO3��������������Ӧ�Ŀ�ʼ��������Ҫ��ע�����������������壮ʵ�鲽��Ϊ��
�ټ��װ�õ������ԣ� �ڳ�ȡ10�˸�����ռ���Ʒ������ƿ�У�D��ע�����ڼ���������ϡ���ᣬȷ����װ��F������320�ˣ�����װ�ã� �۴��ɼ�1����������ע����A 10�Σ�ȷ����װ��F������320.5�ˣ��ܹرյ��ɼ�1��Ȼ���ƶ���������ϡ������ε�����Ʒ�У�ֱ�����ٲ�������Ϊֹ�� �ݴ��ɼ�1����������ע����A10�Σ� ����ȷ��װ��F����Ϊ321.6�ˣ�
����̽����
��i��Eװ�õ������ǣ�Gװ�����ã�
��ii�����������ע�����������ʹ��죬��ⶨ��Na2CO3�����������ᣨ�ƫ����ƫС���������䡱����
��iii���Ը���ʵ���������ݼ�������ռ���Ʒ��Na2CO3������������
���𰸡���������1���������ڿ�����ȼ�����ľ������ɲⶨ����ռ������� ��ʵ������������⣻
��ʵ������������⣻
��2������ʵ���Ҽ��װ�������Ե�ԭ����������װ���ڽ��������Լ���ʱ���ܳ��ֵ�����
��3����I������ʵ���Ҷ�ҩƷȡ��ʱ��Ҫ���������Ƶ����ʣ�����ͼʾ�����д��ڵIJ����ϲ���Ҫ������⣻
��II����������������̼�����������ᷴӦ�����ʣ�д��������Ӧ�Ļ�ѧ����ʽ�������ѡ��ҩƷ��װ�ã��Բ����п��ܳ��ֵĽ�����з����������жϣ�
��III����i�����ݶ�����װ�ü�ʵ��Ŀ�����⣬���װ��������ҩƷ�����ԣ��ж�ʢ��Ũ�����װ��E��ʢ�ż�ʯ�ҵ�װ��G�����ã�
��ii����������ע����������ʵ������ã��Բ�ǡ�����������з������жϸò��������Բⶨ�����Ӱ�죻
��iii��ʵ�����ݵĴ���������װ��F�������仯�ó���Ӧ����������̼��������������һ���������ݷ�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼���Ƶ��������Ӷ���������ռ���Ʒ��Na2CO3������������
����⣺��1������ȼ�ջ�ɰ�ɫ�������������ף��ʿɹ۲쵽�����������̣����ڰ���ȼ�����ľ��������� �����������װ���������������50mL×
�����������װ���������������50mL× =10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ��
=10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ�� ��21%��������
��21%��������
��2����������������ע�����Ļ���ʱ����ƿ�����屻����ע���������װ�����������ã����Ŀ����ͻ�ӳ���©�����벹�䣬��ˣ��ɹ۲쵽����©���¶˹ܿڲ������ݣ���ѡD��
��3����I��ȡ��ҩƷʱ���Լ�ƿ��ƿ��Ӧ�����������ϣ��ڱ�����������ʱ�������������Ƽ������տ����е�ˮ�����⣬�������տ����еĶ�����̼�����ʣ���˱����ܷⱣ�棻
��II���������������ᷢ���кͷ�Ӧ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H2O��̼���������ᷢ�����ֽⷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���壬��ѧ����ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2������Ϊ��ѡ�õ�������лӷ��ԣ����ò����Ķ�����̼���������ⶨ��ȷ�������ɶ�̼�������������ⶨ��ȷ����ѡ��������ݻ����ޣ��������϶�����ʱ���������������ѡע��������ʱ���������������ʵ��ʧ�ܣ����ԣ�����ҩƷʱҪ�ʵ����п��ƣ�
��III����i������װ��E��Ũ���������ˮ�ԣ���װ����ʵ������ҪΪ��ȥ������̼�е�ˮ������װ��G�еļ�ʯ�Ҽ������տ����е�ˮ���������տ����еĶ�����̼��������װ��F�����֮�䣬�������ڷ�ֹ�����еĶ�����̼��ˮ��������F�У�
��ii������ע������ʹװ���ڲ����Ķ�����̼��ȫ��װ��F������������Һ���գ��������ٶȹ��죬����ɶ�����̼����δ�����ն��ų���ʹ�òⶨ�Ķ�����̼����ƫС�����������ⶨ���ƫС��
��iii������װ��F�������仯����֪��Ӧ���ɶ�����̼������=321.6g-320.5g=1.1g������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 1.1g
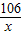 =
=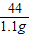 x=2.65g
x=2.65g
���ռ���Ʒ��Na2CO3����������=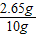 ×100%=26.5%
×100%=26.5%
�ʴ�Ϊ����1�������������̣�10�� ��21%��
��21%��
��2��D��
��3����I�������������ƹ�����ˮ���⣻���������������̼��Ӧ���ʣ�
��II��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ķ�����̼�л���ˮ�������Ȼ������壻����Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣���������
��III����i����ȥ������̼�е�ˮ��������ֹ�����еĶ�����̼��ˮ��������F�У���ii��ƫС����iii��26.5%��
�����������������ɶ�����̼��������ʱ������ʹ����ȫ��Ӧ�������321.6g�뿪ʼ����ҩƷ������320g����������ã���Ϊ����ʵ������и�װ�û�������װ�ÿ����еĶ�����̼�����պ�������Ϊ320.5g��
 ��ʵ������������⣻
��ʵ������������⣻��2������ʵ���Ҽ��װ�������Ե�ԭ����������װ���ڽ��������Լ���ʱ���ܳ��ֵ�����
��3����I������ʵ���Ҷ�ҩƷȡ��ʱ��Ҫ���������Ƶ����ʣ�����ͼʾ�����д��ڵIJ����ϲ���Ҫ������⣻
��II����������������̼�����������ᷴӦ�����ʣ�д��������Ӧ�Ļ�ѧ����ʽ�������ѡ��ҩƷ��װ�ã��Բ����п��ܳ��ֵĽ�����з����������жϣ�
��III����i�����ݶ�����װ�ü�ʵ��Ŀ�����⣬���װ��������ҩƷ�����ԣ��ж�ʢ��Ũ�����װ��E��ʢ�ż�ʯ�ҵ�װ��G�����ã�
��ii����������ע����������ʵ������ã��Բ�ǡ�����������з������жϸò��������Բⶨ�����Ӱ�죻
��iii��ʵ�����ݵĴ���������װ��F�������仯�ó���Ӧ����������̼��������������һ���������ݷ�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼���Ƶ��������Ӷ���������ռ���Ʒ��Na2CO3������������
����⣺��1������ȼ�ջ�ɰ�ɫ�������������ף��ʿɹ۲쵽�����������̣����ڰ���ȼ�����ľ���������
 �����������װ���������������50mL×
�����������װ���������������50mL× =10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ��
=10mL������ע������10mL������벹�䣬ע������ʣ���������20mL-10mL=10mL����ˣ����Կ��������������Ƶ�Լ10mL������ʵ���ٴ���֤�˿�����Լ�� ��21%��������
��21%����������2����������������ע�����Ļ���ʱ����ƿ�����屻����ע���������װ�����������ã����Ŀ����ͻ�ӳ���©�����벹�䣬��ˣ��ɹ۲쵽����©���¶˹ܿڲ������ݣ���ѡD��
��3����I��ȡ��ҩƷʱ���Լ�ƿ��ƿ��Ӧ�����������ϣ��ڱ�����������ʱ�������������Ƽ������տ����е�ˮ�����⣬�������տ����еĶ�����̼�����ʣ���˱����ܷⱣ�棻
��II���������������ᷢ���кͷ�Ӧ�����Ȼ��ƺ�ˮ����ѧ����ʽΪNaOH+HCl=NaCl+H2O��̼���������ᷢ�����ֽⷴӦ�������Ȼ��ơ�ˮ�Ͷ�����̼���壬��ѧ����ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2������Ϊ��ѡ�õ�������лӷ��ԣ����ò����Ķ�����̼���������ⶨ��ȷ�������ɶ�̼�������������ⶨ��ȷ����ѡ��������ݻ����ޣ��������϶�����ʱ���������������ѡע��������ʱ���������������ʵ��ʧ�ܣ����ԣ�����ҩƷʱҪ�ʵ����п��ƣ�
��III����i������װ��E��Ũ���������ˮ�ԣ���װ����ʵ������ҪΪ��ȥ������̼�е�ˮ������װ��G�еļ�ʯ�Ҽ������տ����е�ˮ���������տ����еĶ�����̼��������װ��F�����֮�䣬�������ڷ�ֹ�����еĶ�����̼��ˮ��������F�У�
��ii������ע������ʹװ���ڲ����Ķ�����̼��ȫ��װ��F������������Һ���գ��������ٶȹ��죬����ɶ�����̼����δ�����ն��ų���ʹ�òⶨ�Ķ�����̼����ƫС�����������ⶨ���ƫС��
��iii������װ��F�������仯����֪��Ӧ���ɶ�����̼������=321.6g-320.5g=1.1g������Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 1.1g
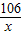 =
=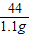 x=2.65g
x=2.65g���ռ���Ʒ��Na2CO3����������=
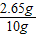 ×100%=26.5%
×100%=26.5%�ʴ�Ϊ����1�������������̣�10��
 ��21%��
��21%����2��D��
��3����I�������������ƹ�����ˮ���⣻���������������̼��Ӧ���ʣ�
��II��NaOH+HCl=NaCl+H2O��Na2CO3+2HCl=2NaCl+H2O+CO2���������Ķ�����̼�л���ˮ�������Ȼ������壻����Ʒȡ���ˣ�����������ܳ���ע�������̣�����Ʒȡ���ˣ���������̫�٣���������
��III����i����ȥ������̼�е�ˮ��������ֹ�����еĶ�����̼��ˮ��������F�У���ii��ƫС����iii��26.5%��
�����������������ɶ�����̼��������ʱ������ʹ����ȫ��Ӧ�������321.6g�뿪ʼ����ҩƷ������320g����������ã���Ϊ����ʵ������и�װ�û�������װ�ÿ����еĶ�����̼�����պ�������Ϊ320.5g��

��ϰ��ϵ�д�
�����Ŀ

 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����