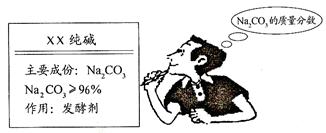
��Ŀ����
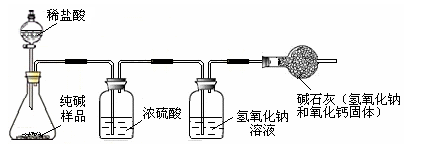
��3�֣�����ʹ�õ���ˮ���ڶ�����һ��ˮ������֪����Ҫ�ɷ���̼��ƺ�������þ����ϡ������������Щˮ����ijУ��ѧ����С��ͬѧ�ڲⶨˮ����̼��Ƶĺ���ʱ����200gˮ���м���������ϡ���ᣬ��ͬʱ������3�����ڲ���������������й��������£�
��ش��������⣺
��1��������һ���������нϴ�������Ӧ��ʱ���� ��
��2��ˮ����̼��Ƶ����������Ƕ��٣�
| ʱ��/s | 0 | 30 | 50 | 90 | 120 | 150 | 180 |
| ��������/g | 0 | 30 | 50 | 60 | 80 | 66 | 66 |
��1��������һ���������нϴ�������Ӧ��ʱ���� ��
��2��ˮ����̼��Ƶ����������Ƕ��٣�
��1��120 ��2��75%
�����������1�����ݱ����е�ǰ�Ĵε����ݷ�����������̼��������Ӧ��������ģ�����120sʱͻȻ�������ּ������ˣ�˵����ʱ��ʱ�IJ����нϴ����
��2������ϡ�����������ģ��ɱ����е������������û���֪����150sʱ̼�������ȫ��Ӧ�ˣ��ɴ˿ɵõ��������壨CO2��������Ϊ66g���ٸ���̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ����̼��Ƶ�������
�⣺��200gˮ����̼��Ƶ�����Ϊx
CaCO3 + 2HCl = CaCl2 + H2O +CO2��
100 44
x 66g

��ã�x=150g
��ˮ����̼��Ƶ�����������
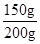 ��100%=75%
��100%=75%��ˮ����̼��Ƶ�����������75%��
�����������ǹ��ڻ�ѧ����ʽ�ļ����⣬��Ҫ������ͼ������Ӧ����ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ��Ҫ�н�ǿ�����ݷ�������������Ĺؼ����ҳ���صĻ�ѧ��Ӧ����������֪����δ֪��Ӧ�������������㣬����Ҫ�淶��

��ϰ��ϵ�д�
�����Ŀ

 ������
������ ������
������