��Ŀ����
ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮| ������Ŀ | ������g�� |
| NaOH��Na2CO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���������������� | 141.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��55�룩 | 149.00 |
�ף�����ϡ�����ң�����KOH��Һ��������Ca��OH��2��Һ��������CaCl2��Һ��
�ڶ�С�����õ�����ƽ�ⶨNaOH��Na2CO3�Ļ������Na2CO3������������װ����ͼ��������ˮ���Ȼ���Ļӷ�����ʵ�����������
��1��д���������������ᷢ����ѧ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����ͨ������������������Na2CO3����������Ϊ���٣�
�� ��

���𰸡��������������ʵ����ʿ���ѡ������ij�ȥ���ʵ����ʣ����ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ��д��ѧ����ʽ�����ݱ������ݺͻ�ѧ����ʽ���Խ�����ط���ļ��㣮
����⣺�����������������ƺ�̼���ƶ���Ӧ������������Һ���ܳ�ȥ̼���ƣ�����Ca��OH��2��Һ��̼������Һ��Ӧ����̼��Ƴ���������������Һ������������Һ���ܺ�����������Һ��Ӧ���Ȼ�����̼���Ʒ�Ӧ�������µ������Ȼ��ƣ��������
��1���������ơ�̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ�ֱ�Ϊ��NaOH+HCl�TNaCl+H2O��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��2���⣺CO2������=141.20g+10.0g-149.0g=2.2g
��ԭ�������Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g

x=5.3g
�������Na2CO3����������Ϊ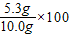 %=53.0%
%=53.0%
�𣺻������Na2CO3����������Ϊ53.0%
������������Ҫ�����˻�ѧ����ʽ����д��ѡ���ȥ���ʵ��Լ�������ݻ�ѧ����ʽ������ؼ���ȷ�������ݣ�ѡ������Լ�ʱ��������ԭ�����ʷ�Ӧ���������ʷ�Ӧʱ���������µ����ʣ�
����⣺�����������������ƺ�̼���ƶ���Ӧ������������Һ���ܳ�ȥ̼���ƣ�����Ca��OH��2��Һ��̼������Һ��Ӧ����̼��Ƴ���������������Һ������������Һ���ܺ�����������Һ��Ӧ���Ȼ�����̼���Ʒ�Ӧ�������µ������Ȼ��ƣ��������
��1���������ơ�̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ�ֱ�Ϊ��NaOH+HCl�TNaCl+H2O��Na2CO3+2HCl�T2NaCl+H2O+CO2����
��2���⣺CO2������=141.20g+10.0g-149.0g=2.2g
��ԭ�������Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
x 2.2g

x=5.3g
�������Na2CO3����������Ϊ
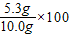 %=53.0%
%=53.0%�𣺻������Na2CO3����������Ϊ53.0%
������������Ҫ�����˻�ѧ����ʽ����д��ѡ���ȥ���ʵ��Լ�������ݻ�ѧ����ʽ������ؼ���ȷ�������ݣ�ѡ������Լ�ʱ��������ԭ�����ʷ�Ӧ���������ʷ�Ӧʱ���������µ����ʣ�

��ϰ��ϵ�д�
 �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д� ��У���˳�̾�ϵ�д�
��У���˳�̾�ϵ�д� ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д�
�����Ŀ
 ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮
ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮��һС���ͬѧȡһ�����ĸù�������ˮ���õ������Һ��Ϊ�˳�ȥ��Һ�е�Na2CO3���ʣ��ס��ҡ���������λͬѧ�ֱ�ѡ�������Լ�����ʵ�飮���к�������
�ף�����ϡ�����ң�����KOH��Һ��������Ca��OH��2��Һ��������CaCl2��Һ��
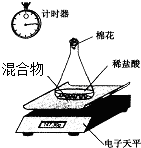
�ڶ�С�����õ�����ƽ�ⶨNaOH��Na2CO3�Ļ������Na2CO3������������װ�ü���ͼ��������ˮ���Ȼ���Ļӷ�����ʵ�����������
| �� �� �� Ŀ | ������g�� |
| NaOH��Na2CO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���������������� | 141.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��55�룩 | 149.00 |
��2����ͨ������������������Na2CO3����������Ϊ���٣�
ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮
��һС���ͬѧȡһ�����ĸù�������ˮ���õ������Һ��Ϊ�˳�ȥ��Һ�е�Na2CO3���ʣ��ס��ҡ���������λͬѧ�ֱ�ѡ�������Լ�����ʵ�飮���к������� ��
�ף�����ϡ���� �ң�����KOH��Һ
��������Ca��OH��2��Һ ��������CaCl2��Һ��
�ڶ�С����������ͼװ�òⶨNaOH��Na2CO3�Ļ������Na2CO3�������������õ�����ƽ�����������±�����������ԭ��������C��12 O��16 Na��23 ��
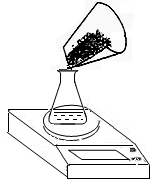
��������Na2CO3����������Ϊ ��
��һС���ͬѧȡһ�����ĸù�������ˮ���õ������Һ��Ϊ�˳�ȥ��Һ�е�Na2CO3���ʣ��ס��ҡ���������λͬѧ�ֱ�ѡ�������Լ�����ʵ�飮���к�������
�ף�����ϡ���� �ң�����KOH��Һ
��������Ca��OH��2��Һ ��������CaCl2��Һ��
�ڶ�С����������ͼװ�òⶨNaOH��Na2CO3�Ļ������Na2CO3�������������õ�����ƽ�����������±�����������ԭ��������C��12 O��16 Na��23 ��

| �������� | ������g�� |
| NaOH��Na2CO3�Ļ���� | 9.30 |
| ��ƿ+ϡ���������������� | 141.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ���� ����Ӧ��ʼ��15�룩 |
148.50 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ���� ����Ӧ��ʼ��35�룩 |
148.30 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ���� ����Ӧ��ʼ��55�룩 |
148.30 |
ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮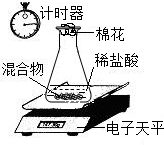
��һС���ͬѧȡһ�����ĸù�������ˮ���õ������Һ��Ϊ�˳�ȥ��Һ�е�Na2CO3���ʣ��ס��ҡ���������λͬѧ�ֱ�ѡ�������Լ�����ʵ�飮���к������� ��
�ף�����ϡ�����ң�����KOH��Һ��������Ca��OH��2��Һ��������CaCl2��Һ��
�ڶ�С�����õ�����ƽ�ⶨNaOH��Na2CO3�Ļ������Na2CO3������������װ����ͼ��������ˮ���Ȼ���Ļӷ�����ʵ�����������
��1��д���������������ᷢ����ѧ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����ͨ������������������Na2CO3����������Ϊ���٣�
�� ��

| ������Ŀ | ������g�� |
| NaOH��Na2CO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���������������� | 141.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��55�룩 | 149.00 |
�ף�����ϡ�����ң�����KOH��Һ��������Ca��OH��2��Һ��������CaCl2��Һ��
�ڶ�С�����õ�����ƽ�ⶨNaOH��Na2CO3�Ļ������Na2CO3������������װ����ͼ��������ˮ���Ȼ���Ļӷ�����ʵ�����������
��1��д���������������ᷢ����ѧ��Ӧ�Ļ�ѧ����ʽ��
��2����ͨ������������������Na2CO3����������Ϊ���٣�
��
 ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮
ij���ʵ�NaOH�����к���Na2CO3����ʦ���ŵ�һС������ȥNaOH��Һ��Na2CO3��ʵ�飮�ڶ�С��ͬѧ���ⶨ�ù���������Na2CO3����������ʵ�飮
��һС���ͬѧȡһ�����ĸù�������ˮ���õ������Һ��Ϊ�˳�ȥ��Һ�е�Na2CO3���ʣ��ס��ҡ���������λͬѧ�ֱ�ѡ�������Լ�����ʵ�飮���к�������________��
�ף�����ϡ�����ң�����KOH��Һ��������Ca��OH��2��Һ��������CaCl2��Һ��
�ڶ�С�����õ�����ƽ�ⶨNaOH��Na2CO3�Ļ������Na2CO3������������װ�ü���ͼ��������ˮ���Ȼ���Ļӷ�����ʵ�����������
| �ơ� ���� � Ŀ | ������g�� |
| NaOH��Na2CO3�Ļ���� | 10.00 |
| ��ƿ+ϡ���������������� | 141.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��15�룩 | 149.20 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��35�룩 | 149.00 |
| ��ƿ+ϡ��������+ȫ��NaOH��Na2CO3�Ļ�����Ӧ��ʼ��55�룩 | 149.00 |
��2����ͨ������������������Na2CO3����������Ϊ���٣�