��Ŀ����
ijУ�о���ѧϰС��õ�һ������Ʒ����֪�����������Ǹ֣���λͬѧ�����һ��ʵ�������м�����������200mlϡ���ᣨ�ܶ�Ϊ1.04g/cm3�����Ĵμ��뵽�������ϣ����ɵ������ܱ���ȫ�ռ��������г�����ʵ�����ݼ�¼���±����˿���Ʒ����ֻ�������͵���̼����������ɵģ�̼�������ᷴӦҲ���������ᣩ��������̼��������Ϊ2%��4.3%����
��ϸ�Ķ������������ݺ�
��1����______�μ������Ტ��ַ�Ӧ����Ʒ�е���������ǡ����ȫ��Ӧ��
��2��ͨ������ش��������Ʒ�е���̼����������Ϊ���٣������������Ǹ֣�
��3�������õ�������Һ��HCl������������
| ���� | ����������� | ����H2�������� | ʣ��������� |
| 1 | 50ml | 0.2g | |
| 2 | 50ml | 0.4g | |
| 3 | 50ml | 0.6g | 0.2g |
| 4 | 50ml | 0.6g | 0.2g |
��1����______�μ������Ტ��ַ�Ӧ����Ʒ�е���������ǡ����ȫ��Ӧ��
��2��ͨ������ش��������Ʒ�е���̼����������Ϊ���٣������������Ǹ֣�
��3�������õ�������Һ��HCl������������
��1������ǰ���μ���ϡ����������H2�Ĺ�ϵ��������150mLϡ���ᣬ������Ʒ����ǡ����ϡ������ȫ��Ӧ��
�ʴ�3��
��2������Ʒ��Fe������Ϊx�����βμӷ�Ӧ��HCl����Ϊy
��������ã�Fe+2HCl=FeCl2+H2��
56 73 2
x y 0.6g
56��2=x��0.6g
73��2=y��0.6g
��֮�ã�x=16.8g y=21.9g
����Ʒ�е���̼����������=
��100%��1.18%
��1.18%��2%��������Ʒ���ڸ�
���������Ʒ�е���̼����������Ϊ1.18%�����Ǹ֣�
��3��������Һ��HCl����������=
��100%��14.04%
�����õ�������Һ��HCl����������Ϊ14.04%��
�ʴ�3��
��2������Ʒ��Fe������Ϊx�����βμӷ�Ӧ��HCl����Ϊy
��������ã�Fe+2HCl=FeCl2+H2��
56 73 2
x y 0.6g
56��2=x��0.6g
73��2=y��0.6g
��֮�ã�x=16.8g y=21.9g
����Ʒ�е���̼����������=
| 0.2g |
| 16.8g+0.2g |
��1.18%��2%��������Ʒ���ڸ�
���������Ʒ�е���̼����������Ϊ1.18%�����Ǹ֣�
��3��������Һ��HCl����������=
| 21.9g |
| 150ml��1.04g/ml |
�����õ�������Һ��HCl����������Ϊ14.04%��

��ϰ��ϵ�д�
�����Ŀ
��1��ijУ�о���ѧϰС��������1000g������������Ϊ15%������������Һ�����������ƹ��� g��ˮ mL��ˮ���ܶ���1g/cm3����������������ʱ ����ܡ����ܡ�������ֽ�ϳ�����
��2�����ұ�����λͬѧ��̽������Һ���̪���õ�ʵ��ʱ��������һ��������������������Һ�����̪��Һ����Һ����˺�ɫ��һ�����ɫ����ʧ�ˣ�
�����롿��
�ף������Ƿ�̪���ʵ�Ե�ʣ�
�ң�����������������Һ������ж�����̼��Ӧ��Ե�ʣ�
���������Ƿ�̪�������������Ӧ��ʹ��ɫ��ʧ��Ե�ʣ�
��������������������Һ����������С�йأ�
�����۷�������
�ټ�ͬѧ������Լ��IJ��룬���������λͬѧ�ķ���λͬѧ�������� ��
����ͬѧ�IJ���Ҳ����ȷ�������� ��
��ʵ����ơ���
��Ϊ֤ʵ��ͬѧ�IJ��룬����������ʵ�飬������±���
��ͨ������ʵ�飬��λͬѧ��������Һ�ȱ�ɺ�ɫ��һ�����ɫ����ʧ����˷�̪��ɫ��ȥ�������أ�����ͬѧ�IJ�����ȷ���������ʵ��֤��������й����⣺
ʵ�鷽�����۲쵽������ͽ��ۣ�
��3����������С����Һ�к�ɫ����ʧ���������������Һ�к�ɫ����ʧ����֤��
��
�������������з�̪��Һ�����������������������Һ��ˮϡ�۲쵽������ͽ��ۣ� ��
��4����8�֣���У�о���ѧϰС�������������Ƶ�15%������������Һ�еμ�ϡ���ᣬ�����ݲ������ɴ˿�֪���õ�NaOH�����ѱ��ʣ�д�����������ڿ����б��ʵĻ�ѧ����ʽ ��������������Ӧ�� ���森��2�֣�
��������⡿�����õ�NaOH���ʳ̶�������
����Ʒ��������ȳ�ȡ21.2g ��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ���һ������������ϡ����ֱ����������������CO2�����������Na2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH������������
������ʵ�顿��ʵ���ü���ϡ��������������CO2�����������ϵ����ͼ��ʾ��
�����ݴ�������д�����¼�����̣�
��5������Ʒ��NaOH����������Ϊ���٣�
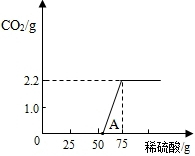
����˼�뽻�������ٴ�ͼ��0��A��˵������NaOH ��Na2CO3�Ļ����Һ�У�����ϡ���ᣬ���ȷ�Ӧ�������� ���ڸ��ݷ�Ӧ����ʽ������NaOH���ֱ��ʻ�ȫ�����ʣ���û�б��ʵ�NaOH��ȣ��кͷ�Ӧʱ����ϡ������� �����ȡ����ڡ���С�ڡ�����2�֣�
��2�����ұ�����λͬѧ��̽������Һ���̪���õ�ʵ��ʱ��������һ��������������������Һ�����̪��Һ����Һ����˺�ɫ��һ�����ɫ����ʧ�ˣ�
�����롿��
�ף������Ƿ�̪���ʵ�Ե�ʣ�
�ң�����������������Һ������ж�����̼��Ӧ��Ե�ʣ�
���������Ƿ�̪�������������Ӧ��ʹ��ɫ��ʧ��Ե�ʣ�
��������������������Һ����������С�йأ�
�����۷�������
�ټ�ͬѧ������Լ��IJ��룬���������λͬѧ�ķ���λͬѧ��������
����ͬѧ�IJ���Ҳ����ȷ��������
��ʵ����ơ���
��Ϊ֤ʵ��ͬѧ�IJ��룬����������ʵ�飬������±���
| ʵ�鲽�� | �����һ�����Ŀ�� |
| 1������й�������ˮ��������������Һ�� | |
| 2��������������Һ�е����̪�������Ϸ���һЩֲ���ͣ� |
ʵ�鷽�����۲쵽������ͽ��ۣ�
��3����������С����Һ�к�ɫ����ʧ���������������Һ�к�ɫ����ʧ����֤��
�������������з�̪��Һ�����������������������Һ��ˮϡ�۲쵽������ͽ��ۣ�
��4����8�֣���У�о���ѧϰС�������������Ƶ�15%������������Һ�еμ�ϡ���ᣬ�����ݲ������ɴ˿�֪���õ�NaOH�����ѱ��ʣ�д�����������ڿ����б��ʵĻ�ѧ����ʽ
��������⡿�����õ�NaOH���ʳ̶�������
����Ʒ��������ȳ�ȡ21.2g ��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ���һ������������ϡ����ֱ����������������CO2�����������Na2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH������������
������ʵ�顿��ʵ���ü���ϡ��������������CO2�����������ϵ����ͼ��ʾ��
�����ݴ�������д�����¼�����̣�
��5������Ʒ��NaOH����������Ϊ���٣�

����˼�뽻�������ٴ�ͼ��0��A��˵������NaOH ��Na2CO3�Ļ����Һ�У�����ϡ���ᣬ���ȷ�Ӧ��������

 ��2010?�Ű���ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
��2010?�Ű���ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽���� ��ȷ��ǻ�ѧ�о����ʵ���Ҫ����֮һ������Ļ�ѧ������̼�����ƣ���֪̼���ֽ⣬����������������Ҳ�ֽ⣬���������������ijУ�о���ѧϰС��Դ�չ��̽����
��ȷ��ǻ�ѧ�о����ʵ���Ҫ����֮һ������Ļ�ѧ������̼�����ƣ���֪̼���ֽ⣬����������������Ҳ�ֽ⣬���������������ijУ�о���ѧϰС��Դ�չ��̽����