��Ŀ����
ʵ��������ͼ��ʾװ������ȡ���ռ�������̼����
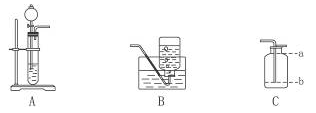
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_____________________��
��2���ռ�CO2ʱ������Bװ�õ�ԭ����_____________________����Cװ�����ռ�CO2ʱ���ܿ��¶�λ��Ӧ��__________��ѡ�a����b���������������������Ƿ�ΪCO2�ķ�Ӧԭ���ǣ��û�ѧ����ʽ��ʾ��__________________��
��3����ʵ����Ҫ���мס���������ѧ��ȤС������װ�������¸Ľ���
���飺���Թܻ�����ƿ�����ʵ���˫���������Ա��Ƶý϶�����CO2��
���飺������©��������ͨ©������ʹ���������㡣
����Ϊ���Ͽ��е���__________��ķ�����
��2���ռ�CO2ʱ������Bװ�õ�ԭ����_____________________����Cװ�����ռ�CO2ʱ���ܿ��¶�λ��Ӧ��__________��ѡ�a����b���������������������Ƿ�ΪCO2�ķ�Ӧԭ���ǣ��û�ѧ����ʽ��ʾ��__________________��
��3����ʵ����Ҫ���мס���������ѧ��ȤС������װ�������¸Ľ���
���飺���Թܻ�����ƿ�����ʵ���˫���������Ա��Ƶý϶�����CO2��
���飺������©��������ͨ©������ʹ���������㡣
����Ϊ���Ͽ��е���__________��ķ�����
��1��CaCO3+2HCl====CaCl2+H2O+CO2��
��2��CO2������ˮ ��b ��Ca (OH )2+ CO2====CaCO3��+H2O
��3����
��2��CO2������ˮ ��b ��Ca (OH )2+ CO2====CaCO3��+H2O
��3����

��ϰ��ϵ�д�
�����Ŀ
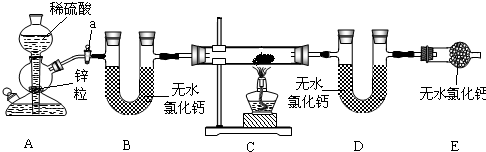

 ʵ��������ͼ��ʾװ����ȡCO2������֤CO2�����ʣ��Իش��������⣺
ʵ��������ͼ��ʾװ����ȡCO2������֤CO2�����ʣ��Իش��������⣺
 ʵ��������ͼ��ʾװ���Ʊ���ƿ������������ʵ�飮
ʵ��������ͼ��ʾװ���Ʊ���ƿ������������ʵ�飮