��Ŀ����
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ�______��
��2������ǿ������õ�pHС��7�������ɣ�______��
������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˣ���С����������������ԭ���������²��룺�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ���ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ��
��1��Ϊ��֤����٣�����ͬѧ��������ʵ�飺�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ����______��ʵ����������̪��Һ��ɫ��ʧ������е������أ�
��2��Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���ɴ˿ɵó�����������ۣ�����1��˵��Na2CO3��Һ��______�ԣ�����2��˵����̪��Һ��ɫ��ʧ������еĶ�����̼�أ�
��3����С��ͬѧͨ���������ϵ�֪��������������ҺŨ�Ⱥܴ�ʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���ʵ�鷽��______���ڹ۲쵽������______��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ�����
�ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t���������
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
��1������x=______��
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����______�ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
��3������ʵ���е�ϸ�ں������������ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ����______����ʵ������У�Ҫ�ò��������Ͻ��裬��������Ŀ����______������ʵ����������ⷢ�������ݳ��֣�����Ϊԭ����______���ܷ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH������Ϊ���ڿ�����______�� �ۿ�����______��
��4��Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g����
��1����Ʒ���������Ƶ�������______
��2�����������Ʒ�Ӧ�������������______
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ��

����֪Na2CO3+2HCl�T2NaCl+H2O+CO2����

���𰸡�����������һ����1����ȷ����pH��ֽ�ⶨ��Һ��pH��
��2���ڵμӹ��������û��Ӧ�����۽���������ϡ�͵�ʲô�̶ȶ�������С��7��
��������1��Ϊ����֤�Dz���������Ӱ�죬����Ҫ�ų������ĸ��ţ�
��2���������ƺͶ�����̼��Ӧ����̼���ƣ���̼����Ҳ�ܹ��Ƿ�̪��죬���ų������ֿ��ܣ�
����������1�����ݱ��е����ݽ��н��
��2�������кͷ�Ӧ�Ƿ��ȷ�Ӧ���з�����
��3������ʵ���еIJ���������ɵ�Ӱ��ͺ���������⣻
��4�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼��������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ��������⣮
����⣺����һ
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ��ò�����պȡ��Һ����PH��ֽ�ϣ��������ɫ�����վͿ���֪����Һ��PH��
��2����Ϊϡ������������Һʱ�����Լ�������Һ��PH��С��ֻ�����Ľӽ���7�����Dz��ܵ��ڻ�С��7�����PHС��7��˵���������ƺ����ᷢ���˻�ѧ��Ӧ��
������
��1��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���ǣ���ȥ��Һ���ܽ�����������ų������ĸ��ţ�
��2������1�����Լ��Ե�������ʹ��̪��Һ���ɫ��˵��̼������Һ�Լ��ԣ�
��3��������������Ϣ����֪������������������Һϡ�ͺ��ٵμӷ�̪�۲���������ʾ��ɫ��˵����ȡ����ҺŨ�ȹ���
������
��1���Ƚϵ�����͵�һ�������е�������������Ƶ�Ũ�ȿ���֪�������������������ƺ������Ũ�ȶ��ǵ�һ���2�������¶ȵı仯�ǵ�һ���4�����ڶ������һ��Ƚϣ�����������������䣬���������Ƶ�Ũ���ǵ�һ���2���������¶ȵĸı�Ҳ�ǵ�һ���2��������X=7
��2���������ƺ����ᷢ���кͷ�Ӧ����Һ���¶Ȼ����ߣ�������ƿ�ڵ�ѹǿ����ʹU�ιܵ�Һ����ָ߶Ȳ
��3������������������Ʒ�Ӧʱ������֪������μ��ٶȹ�����ܻ��������������
���ò����������ܹ���������������Ƴ�ַ�Ӧ��
��������������˵���������������Ʊ��ʣ�
�������������̼���ƣ����Կ��Եó�����ɷֿ�����̼���ƣ�̼���ƺ��������ƵȲ���
��4����1����2�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼����������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ����⣻
��3�������Ⱥ��������Ʒ�Ӧ��Ȼ��ź�̼���Ʒ�Ӧ���ɶ�����̼
�ʴ�Ϊ������һ����1����PH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������ֽ�ϣ�����ֽ��ɫ���ɫ�����ն���pH��
��2���ų���ϡ�Ͷ�ʹ��ҺpH��С�����أ�
����������1������������
��2���
��3����ȡ������Һ��ˮϡ�ͺ��ٵμ���ɫ��̪��
����Һ�ʺ�ɫ��
����������1��7����2��U����Һ������Ҹߣ�
��3���ٷ�ֹϡ�������
��ʹ��Ӧ���
������������Һ�к���̼����
��Na2CO3NaOH��Na2CO3�Ļ���
��4���⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y×10% 2.2g

��ã�x=5.3g y=36.5g
NaOH��������13.3g-5.3g=8g
����������Ʒ�Ӧ�����������Ϊz
NaOH+HCl�TNaCl+H2O��0.5�֣�
40 36.5
8g z×10%

��� z=73g
�𣺣�1����Ʒ���������Ƶ�����Ϊ8g��
��2�����������Ʒ�Ӧ�����������Ϊ73g��
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ����

������������Ҫ������ϡ���������������Һ�����кͷ�Ӧ�������ʵ�������ط���֤���ȷ�������ݣ����������кͷ�Ӧ�ĸ����Ӧ�ã�����pH��ֽ�ⶨ��Һ��pHʱ�IJ���������
��2���ڵμӹ��������û��Ӧ�����۽���������ϡ�͵�ʲô�̶ȶ�������С��7��
��������1��Ϊ����֤�Dz���������Ӱ�죬����Ҫ�ų������ĸ��ţ�
��2���������ƺͶ�����̼��Ӧ����̼���ƣ���̼����Ҳ�ܹ��Ƿ�̪��죬���ų������ֿ��ܣ�
����������1�����ݱ��е����ݽ��н��
��2�������кͷ�Ӧ�Ƿ��ȷ�Ӧ���з�����
��3������ʵ���еIJ���������ɵ�Ӱ��ͺ���������⣻
��4�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼��������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ��������⣮
����⣺����һ
��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ��ò�����պȡ��Һ����PH��ֽ�ϣ��������ɫ�����վͿ���֪����Һ��PH��
��2����Ϊϡ������������Һʱ�����Լ�������Һ��PH��С��ֻ�����Ľӽ���7�����Dz��ܵ��ڻ�С��7�����PHС��7��˵���������ƺ����ᷢ���˻�ѧ��Ӧ��
������
��1��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���ǣ���ȥ��Һ���ܽ�����������ų������ĸ��ţ�
��2������1�����Լ��Ե�������ʹ��̪��Һ���ɫ��˵��̼������Һ�Լ��ԣ�
��3��������������Ϣ����֪������������������Һϡ�ͺ��ٵμӷ�̪�۲���������ʾ��ɫ��˵����ȡ����ҺŨ�ȹ���
������
��1���Ƚϵ�����͵�һ�������е�������������Ƶ�Ũ�ȿ���֪�������������������ƺ������Ũ�ȶ��ǵ�һ���2�������¶ȵı仯�ǵ�һ���4�����ڶ������һ��Ƚϣ�����������������䣬���������Ƶ�Ũ���ǵ�һ���2���������¶ȵĸı�Ҳ�ǵ�һ���2��������X=7
��2���������ƺ����ᷢ���кͷ�Ӧ����Һ���¶Ȼ����ߣ�������ƿ�ڵ�ѹǿ����ʹU�ιܵ�Һ����ָ߶Ȳ
��3������������������Ʒ�Ӧʱ������֪������μ��ٶȹ�����ܻ��������������
���ò����������ܹ���������������Ƴ�ַ�Ӧ��
��������������˵���������������Ʊ��ʣ�
�������������̼���ƣ����Կ��Եó�����ɷֿ�����̼���ƣ�̼���ƺ��������ƵȲ���
��4����1����2�������������������Ļ�ѧ��Ӧ������֪����̼���ƺ�����ķ�Ӧ��Ȼ����ݶ�����̼����������������̼���Ƶ�������Ȼ���ж��Ƿ����������ƽ����⣻
��3�������Ⱥ��������Ʒ�Ӧ��Ȼ��ź�̼���Ʒ�Ӧ���ɶ�����̼
�ʴ�Ϊ������һ����1����PH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������ֽ�ϣ�����ֽ��ɫ���ɫ�����ն���pH��
��2���ų���ϡ�Ͷ�ʹ��ҺpH��С�����أ�
����������1������������
��2���
��3����ȡ������Һ��ˮϡ�ͺ��ٵμ���ɫ��̪��
����Һ�ʺ�ɫ��
����������1��7����2��U����Һ������Ҹߣ�
��3���ٷ�ֹϡ�������
��ʹ��Ӧ���
������������Һ�к���̼����
��Na2CO3NaOH��Na2CO3�Ļ���
��4���⣺�������Ʒ��̼���Ƶ�����Ϊx����̼���Ʒ�Ӧ�����������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x y×10% 2.2g

��ã�x=5.3g y=36.5g
NaOH��������13.3g-5.3g=8g
����������Ʒ�Ӧ�����������Ϊz
NaOH+HCl�TNaCl+H2O��0.5�֣�
40 36.5
8g z×10%

��� z=73g
�𣺣�1����Ʒ���������Ƶ�����Ϊ8g��
��2�����������Ʒ�Ӧ�����������Ϊ73g��
��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ����

������������Ҫ������ϡ���������������Һ�����кͷ�Ӧ�������ʵ�������ط���֤���ȷ�������ݣ����������кͷ�Ӧ�ĸ����Ӧ�ã�����pH��ֽ�ⶨ��Һ��pHʱ�IJ���������

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮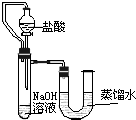
I��ʵ�鷽��
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨNaOH��ҺpHʱ����ȷ�IJ����ǣ� ��
��2������ǿ������õ�pHС��7�������ɣ� ��
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������
NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10�˻�ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
��1������x= ��
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� �ж�NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ��
II��ʵ���е��������
��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3��
��1��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м��������� ��Һ��������ɫ����������Ȼ�����ϲ���Һ�м����̪��Һ��������Һ�ʺ�ɫ����֤�˲��������ȷ�ģ�
��2��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ������ͼ��ʾ��ʵ�飮
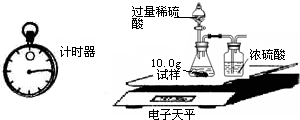
ʵ�����ݼ�¼���£�
��ͨ�������������ݼ���������Ʒ�и��ɷݵ�����������д��������̣��� ��
��3����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ���� ��

I��ʵ�鷽��
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ��
��1����pH��ֽ�ⶨNaOH��ҺpHʱ����ȷ�IJ����ǣ�
��2������ǿ������õ�pHС��7�������ɣ�
����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������
NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ��
����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10�˻�ϣ����¶ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t�����±�����
| ��� | ���� | NaOH��Һ | ��t/�� |
| 1 | 3.65% | 2.00% | 3.5 |
| 2 | 3.65% | 4.00% | x |
| 3 | 7.30% | 8.00% | 14 |
��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ����
II��ʵ���е��������
��ʵ������У����Ƿ���ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ������������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²��룺�ٿ�����NaOH���ڿ�����Na2CO3�� �ۿ�����NaOH��Na2CO3��
��1��С��ͬѧȡ��ɫ��ĩ����������ˮ��������Һ�м���������
��2��Ϊ�˽�һ���о�����λͬѧȡ��10.0g������Ʒ�����õ�����ƽ��ͬ������ͼ��ʾ��ʵ�飮

ʵ�����ݼ�¼���£�
| �� �� �� Ŀ | �� �� ʱ �� | ������g�� |
| ���� | 10.00 | |
| װ��+ϡ�������� | 241.20 | |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��15�� | 249.20 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��35�� | 249.00 |
| װ��+ϡ��������+���� | ��Ӧ��ʼ��55�� | 249.00 |
��3����ͬѧ���������ʵ�������������Na2CO3����������ƫС����ͬѧ�������ǣ�ʵ���������ȷ����


 ����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮