��Ŀ����
̼�и��Ӵ�ļ��塣
��1��̼���ʾ��ж��ص����ʺ���;���������¿ո���д�������ƣ�
����Ȼ���ڵ���Ӳ������ ����Ǧ��о����Ҫ�ɷ�֮һ ��
��2��̼�Ͳ���̼�Ļ������ת����ϵ����ͼ��ʾ��
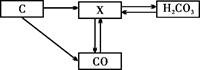
������X�Ļ�ѧʽΪ ��
�������ʵķ����У�CO���� �������ţ�
��̼����Һ��pHֵ 7�������������=������
��3������л����Ǽ��飬��ȼ�յĻ�ѧ��Ӧ����ʽΪ ���������õ����ĺϳ��л��߷��Ӳ����� �����֡�
��4������̼���á��ĺ����ǽ��ܼ��š�����ͼ�пɿ�����
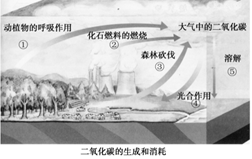
�ٵ��¿����ж�����̼���ӵ������� ����д��ţ���
��ͨ���������Ķ�����̼�Ļ�ѧ��Ӧ����ʽΪ��
��
������ЧӦ�ĺ���� ��дһ������
��1��̼���ʾ��ж��ص����ʺ���;���������¿ո���д�������ƣ�
����Ȼ���ڵ���Ӳ������ ����Ǧ��о����Ҫ�ɷ�֮һ ��
��2��̼�Ͳ���̼�Ļ������ת����ϵ����ͼ��ʾ��

������X�Ļ�ѧʽΪ ��
�������ʵķ����У�CO���� �������ţ�
| A���� | B���� | C���� | D�������� |
��3������л����Ǽ��飬��ȼ�յĻ�ѧ��Ӧ����ʽΪ ���������õ����ĺϳ��л��߷��Ӳ����� �����֡�
��4������̼���á��ĺ����ǽ��ܼ��š�����ͼ�пɿ�����

�ٵ��¿����ж�����̼���ӵ������� ����д��ţ���
��ͨ���������Ķ�����̼�Ļ�ѧ��Ӧ����ʽΪ��
��
������ЧӦ�ĺ���� ��дһ������
��10�֣���1���ٽ��ʯ ��ʯī ��2����CO2 ��D ��<
��3��CH4 + 2O2 CO2 + 2H2O ���ϡ��ϳ����ϳ���ά��
CO2 + 2H2O ���ϡ��ϳ����ϳ���ά��
��4���٢ڢ� CO2 + H2O ==H2CO3 �������ߵ��±����ۻ����������ɣ�
��3��CH4 + 2O2
 CO2 + 2H2O ���ϡ��ϳ����ϳ���ά��
CO2 + 2H2O ���ϡ��ϳ����ϳ���ά����4���٢ڢ� CO2 + H2O ==H2CO3 �������ߵ��±����ۻ����������ɣ�
��1�����ɽ��ʯ��̼ԭ���γ���������ṹ������Ȼ���ڵ���Ӳ�����ʣ�Ǧ��о��ʯī��ճ����϶��Ƴɣ����Ǧ��о����Ҫ�ɷ�֮һΪʯī��
��2������ת����ϵͼ������X����̼���һ����̼ʵ���ת�������ݶ�����̼�����ʼ��仯���ɿ��ƶ�XΪ������̼���仯ѧʽΪCO2��
��һ����̼��̼��������Ԫ����ɣ����ڻ������е������
��̼��Ϊ�������ʣ�����Һ�����ԣ�pH��7��
��3��������̼��������Ԫ����ɣ�ȼ��ʱ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2 CO2+2H2O���ϳɲ��ϰ������ϡ��ϳ���ά���ϳ��������л��߷��Ӳ��ϣ��������ж��н�Ϊ�㷺��ʹ�ã�
CO2+2H2O���ϳɲ��ϰ������ϡ��ϳ���ά���ϳ��������л��߷��Ӳ��ϣ��������ж��н�Ϊ�㷺��ʹ�ã�
��4�����ݶ�����̼����������ͼƬ��ͼ�Тٶ�ֲ��ĺ������á��ڻ�ʯȼ�ϵ�ȼ�ա���ɭ�ֿ��������¿����ж�����̼���ӣ�������̼��������ˮ������ˮ��Ӧ����̼�ᣬ���ͨ���������Ķ�����̼�Ļ�ѧ��Ӧ����ʽΪCO2+H2O�TH2CO3������ЧӦ���µ����������߶�ʹ�����ڻ�����ƽ�����ߵȡ�
��2������ת����ϵͼ������X����̼���һ����̼ʵ���ת�������ݶ�����̼�����ʼ��仯���ɿ��ƶ�XΪ������̼���仯ѧʽΪCO2��
��һ����̼��̼��������Ԫ����ɣ����ڻ������е������
��̼��Ϊ�������ʣ�����Һ�����ԣ�pH��7��
��3��������̼��������Ԫ����ɣ�ȼ��ʱ���ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2
 CO2+2H2O���ϳɲ��ϰ������ϡ��ϳ���ά���ϳ��������л��߷��Ӳ��ϣ��������ж��н�Ϊ�㷺��ʹ�ã�
CO2+2H2O���ϳɲ��ϰ������ϡ��ϳ���ά���ϳ��������л��߷��Ӳ��ϣ��������ж��н�Ϊ�㷺��ʹ�ã���4�����ݶ�����̼����������ͼƬ��ͼ�Тٶ�ֲ��ĺ������á��ڻ�ʯȼ�ϵ�ȼ�ա���ɭ�ֿ��������¿����ж�����̼���ӣ�������̼��������ˮ������ˮ��Ӧ����̼�ᣬ���ͨ���������Ķ�����̼�Ļ�ѧ��Ӧ����ʽΪCO2+H2O�TH2CO3������ЧӦ���µ����������߶�ʹ�����ڻ�����ƽ�����ߵȡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
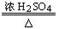 CO��+H2O��ʵ���ҿ�������ͼװ����ȡCO����ԭCuO������˵����
CO��+H2O��ʵ���ҿ�������ͼװ����ȡCO����ԭCuO������˵����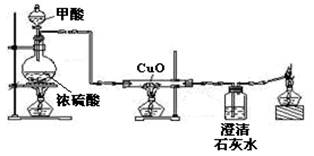
 �����ˮ����������ƿ�ǣ�������������Ϊ ��
�����ˮ����������ƿ�ǣ�������������Ϊ ��
