��Ŀ����
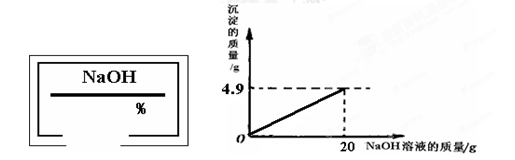
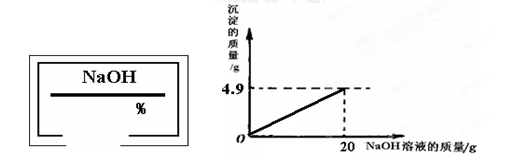
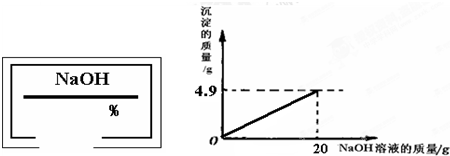
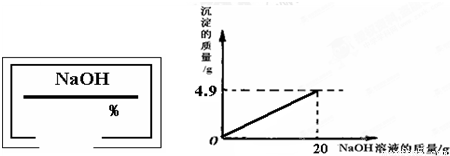
С��ͬѧ��ʵ���ҿ�������������ͭ��ϡ���ᷴӦ��ʵ�����ʵ��������һƿ��ǩ��ȱ����ͼ��ʾ����NaOH��Һ��Ϊ�˲ⶨ����Һ������������������������������ͭ��ϡ���ᷴӦ��ķ�Һ��������Һ���ˣ�Ȼ��ȡ100g��Һ�������μӴ�NaOH��Һ������NaOH��Һ�����������ɳ��������Ĺ�ϵ��ͼ��ʾ��
��1������100g��Һ��CuSO4��������
��2�����������������Һ�����ʵ�����������

��1������100g��Һ��CuSO4��������
��2�����������������Һ�����ʵ�����������
��������1����ͼʾ��֪����������������Һ��20gʱ�����ɳ���������Ϊ4.9g����������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ����100g��Һ��CuSO4��������
��2�����ݻ�ѧ����ʽ�г�����ʽ���Ϳɼ��������CuSO4��Ӧ����4.9g������NaOH������Ȼ��������������������㷽�����㼴�ɣ�
��2�����ݻ�ѧ����ʽ�г�����ʽ���Ϳɼ��������CuSO4��Ӧ����4.9g������NaOH������Ȼ��������������������㷽�����㼴�ɣ�
����⣺��1����100 g��Һ��CuSO4������Ϊx������4.9g������NaOH����Ϊy
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 80 98
x y 4.9g
=
=
x=8g y=4g
��2��������������Һ�����ʵ���������=
��100%=20%
�𣺣�1��100g��Һ��CuSO4��������8g�� ��2��������������Һ�����ʵ�����������20%��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 80 98
x y 4.9g
| 160 |
| x |
| 98 |
| 4.9g |
| 80 |
| y |
x=8g y=4g
��2��������������Һ�����ʵ���������=
| 4g |
| 20g |
�𣺣�1��100g��Һ��CuSO4��������8g�� ��2��������������Һ�����ʵ�����������20%��
������������һ�����������⣬����ؼ���Ҫ������ͬ�ɷֲ���ķ�Ӧ����Ӧ�����ݣ���������û�ѧ����ʽ���м���ʱҪע����ⲽ��Ĺ淶�ԣ�

��ϰ��ϵ�д�
�����Ŀ