��Ŀ����
17������ͬѧ��ѧϰ�������ļʱ������������⣺
�ټ�������������̼Ϊʲôһ���ó���ʯ��ˮ������NaOH��Һ��
��ʵ��ʱ���ս϶��CO2Ϊʲôһ����NaOH��Һ�����ó���ʯ��ˮ��
[��������]a.20��ʱ����ʯ�ҵ��ܽ��Ϊ0.17g��b����ʯ�ҵ��ܽ�������¶����߶����ͣ�
[ʵ��̽��]�ڽ�ʦָ���£�����ͬѧ����������ʵ��̽����
̽��һ�����ڶ�����̼�ļ���
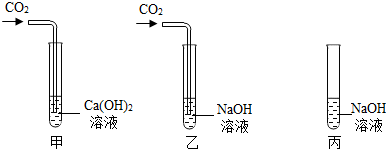
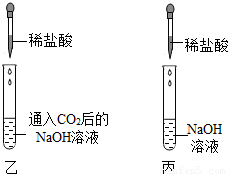
���ڼס��ҡ�����֧�Թ��м������NaOH��Ca��OH��2 ��Һ������ͼ�����ڼס����зֱ�ͨ������CO2��
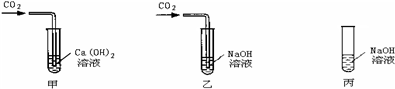
����ʵ�����Һͱ����ֳ�����Һ�У��ֱ��������ϡ���ᣨ����ͼ����

�ش����⣺
��1�����мס��Ҷ�֧�Թܵ�����ֱ���
��2���������Թܿɹ۲쵽�����ݲ�����д���÷�Ӧ�Ļ�ѧ����ʽ��
[�ó�����]��������CO2һ����ʯ��ˮ������NaOH��Һ��
̽���������ڶ�����̼������
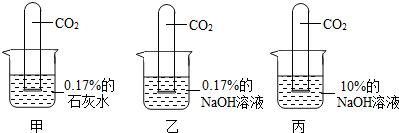
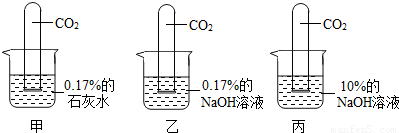
��������ͼ��ʾʵ�飺
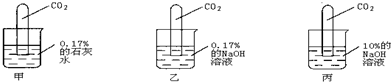
[ʵ������]���Թܣ�Һ���������٣� ���Թܣ�Һ�������ϼ��Ըߣ�
���Թܣ������ӽ���ȫ�����ڹܵ�����һС���ݣ�
��3��С����Ϊ����ֱ������10%��ʯ��ˮ�������0.17%��ʯ��ˮ��������жԱ�ʵ�飮��ͬ��С�յĹ۵��𣿴�
[�ó�����]ʵ��ʱ����CO2ѡ�ý�Ũ��NaOH��Һ������Ca��OH��2��Һ��������
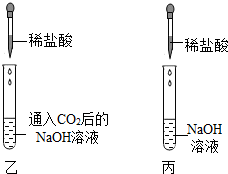
[ʵ����չ]Ϊ��ֱ�۵ع۲쵽CO2��NaOH��Ӧ���������������ͼװ�ý���ʵ�飮����NaOH��Һ�ĵ��룬�ɹ۲쵽��������
�ټ�������������̼Ϊʲôһ���ó���ʯ��ˮ������NaOH��Һ��
��ʵ��ʱ���ս϶��CO2Ϊʲôһ����NaOH��Һ�����ó���ʯ��ˮ��
[��������]a.20��ʱ����ʯ�ҵ��ܽ��Ϊ0.17g��b����ʯ�ҵ��ܽ�������¶����߶����ͣ�
[ʵ��̽��]�ڽ�ʦָ���£�����ͬѧ����������ʵ��̽����
̽��һ�����ڶ�����̼�ļ���
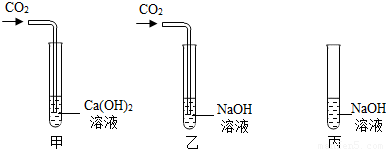
���ڼס��ҡ�����֧�Թ��м������NaOH��Ca��OH��2 ��Һ������ͼ�����ڼס����зֱ�ͨ������CO2��

����ʵ�����Һͱ����ֳ�����Һ�У��ֱ��������ϡ���ᣨ����ͼ����

�ش����⣺
��1�����мס��Ҷ�֧�Թܵ�����ֱ���
���Թܲ�����ɫ�����������ǣ������Թ�����������
����2���������Թܿɹ۲쵽�����ݲ�����д���÷�Ӧ�Ļ�ѧ����ʽ��
Na2CO3+2HCl�T2NaCl+CO2��+H2O
����Ʊ��Թ�ʵ���Ŀ�����������ԹܵĶԱ�ʵ�飬֤�����Թ���CO2��NaOH�����˻�ѧ��Ӧ
��[�ó�����]��������CO2һ����ʯ��ˮ������NaOH��Һ��
̽���������ڶ�����̼������
��������ͼ��ʾʵ�飺

[ʵ������]���Թܣ�Һ���������٣� ���Թܣ�Һ�������ϼ��Ըߣ�
���Թܣ������ӽ���ȫ�����ڹܵ�����һС���ݣ�
��3��С����Ϊ����ֱ������10%��ʯ��ˮ�������0.17%��ʯ��ˮ��������жԱ�ʵ�飮��ͬ��С�յĹ۵��𣿴�
��ͬ��
���ͬ�⡱��ͬ�⡱����������20��ʱ����ʯ�ҵ��ܽ����0.17g�������Ƴ�10%��ʯ��ˮ����20��ʱ0.17%��ʯ��ˮ�DZ�����Һ��ʯ��ˮ�������������������ܴﵽ10%������������Ҳ���֣�
��4���Ƚ��ҡ����н����Թ���Һ��������ó��Ľ�����NaOH��Һ��������������Խ������CO2����Խǿ
��[�ó�����]ʵ��ʱ����CO2ѡ�ý�Ũ��NaOH��Һ������Ca��OH��2��Һ��������
����ʱҪ��������CO2��NaOH��������ˮ���γɵ���ҺŨ�ȴ��������࣬Ч��ߣ���Ca��OH��2�ܣ�����������CO2Ҫ�������Һ
��[ʵ����չ]Ϊ��ֱ�۵ع۲쵽CO2��NaOH��Ӧ���������������ͼװ�ý���ʵ�飮����NaOH��Һ�ĵ��룬�ɹ۲쵽��������
С�������ʹ�
����������1��������̼������������Һ��Ӧʱ�����ɲ�����ˮ�İ�ɫ������������̼������������Һ��Ӧʱ����������
��2��̼���������ᷴӦ������ų������Թ���������������Һ�����ᷴӦ���������ɣ�
��3��Ca��OH��2���ܽ�Ⱥ�С��20��ʱ����ʯ�ҵ��ܽ����0.17g
��4������������������ʵ�����������Һ������������Һ���ն�����̼ʱ�����ն�����̼�����ϴ��������������Һ�������������Ƶ��ܽ������ܲ��ʵ�ʲ��������л�����������������Һ���ն�����̼������������Һ���ն�����̼��Ч����ʯ��ˮ�ã�
��2��̼���������ᷴӦ������ų������Թ���������������Һ�����ᷴӦ���������ɣ�
��3��Ca��OH��2���ܽ�Ⱥ�С��20��ʱ����ʯ�ҵ��ܽ����0.17g
��4������������������ʵ�����������Һ������������Һ���ն�����̼ʱ�����ն�����̼�����ϴ��������������Һ�������������Ƶ��ܽ������ܲ��ʵ�ʲ��������л�����������������Һ���ն�����̼������������Һ���ն�����̼��Ч����ʯ��ˮ�ã�
����⣺��1��������̼���������Ʒ�Ӧ������̼��Ƴ�����ˮ����Ӧ�Ļ�ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��������̼���������Ʒ�Ӧ������̼���ƺ�ˮ
����CO2��ʯ��ˮ����������ʵ��������NaOH������������������Թܲ�����ɫ�����������ǣ������Թ�����������
��2�����Թ������ȶ�����̼���������Ʒ�Ӧ������̼���ƣ�̼���������ᷴӦ������ų������Թ���������������Һ�����ᷴӦ���������ɣ������Թ���������ų���˵���˶�����̼���������Ʒ�Ӧ�����������ʣ����Na2CO3+2HCl�T2NaCl+CO2��+H2O���������ԹܵĶԱ�ʵ�飬֤�����Թ���CO2��NaOH�����˻�ѧ��Ӧ
��3����ΪCa��OH��2���ܽ�Ⱥ�С�����γɵ�ʯ��ˮ�����ʵ�����������С����ֱ������10%��ʯ��ˮ�������ͬ�⣻20��ʱ����ʯ�ҵ��ܽ����0.17g�������Ƴ�10%��ʯ��ˮ
��4����ΪNaOH������ˮ���ܽ�ö࣬��Һ���������������ʹ�����CO2������Խǿ��Ca��OH��2���ܽ�Ⱥ�С���ܽ���٣���Һ����������������С������CO2������Խǿ����
���NaOH��Һ��������������Խ������CO2����Խǿ������ʱҪ��������CO2��NaOH��������ˮ���γɵ���ҺŨ�ȴ��������࣬Ч��ߣ���Ca��OH��2�ܣ�����������CO2Ҫ�������Һ
[ʵ����չ]��������̼��������������������ڲ�ѹǿ��С�����С�������ʹ�
����CO2��ʯ��ˮ����������ʵ��������NaOH������������������Թܲ�����ɫ�����������ǣ������Թ�����������
��2�����Թ������ȶ�����̼���������Ʒ�Ӧ������̼���ƣ�̼���������ᷴӦ������ų������Թ���������������Һ�����ᷴӦ���������ɣ������Թ���������ų���˵���˶�����̼���������Ʒ�Ӧ�����������ʣ����Na2CO3+2HCl�T2NaCl+CO2��+H2O���������ԹܵĶԱ�ʵ�飬֤�����Թ���CO2��NaOH�����˻�ѧ��Ӧ
��3����ΪCa��OH��2���ܽ�Ⱥ�С�����γɵ�ʯ��ˮ�����ʵ�����������С����ֱ������10%��ʯ��ˮ�������ͬ�⣻20��ʱ����ʯ�ҵ��ܽ����0.17g�������Ƴ�10%��ʯ��ˮ
��4����ΪNaOH������ˮ���ܽ�ö࣬��Һ���������������ʹ�����CO2������Խǿ��Ca��OH��2���ܽ�Ⱥ�С���ܽ���٣���Һ����������������С������CO2������Խǿ����
���NaOH��Һ��������������Խ������CO2����Խǿ������ʱҪ��������CO2��NaOH��������ˮ���γɵ���ҺŨ�ȴ��������࣬Ч��ߣ���Ca��OH��2�ܣ�����������CO2Ҫ�������Һ
[ʵ����չ]��������̼��������������������ڲ�ѹǿ��С�����С�������ʹ�
�����������Ĺؼ���Ҫ�����������ƺ��������������ᡢ������̼��Ӧ�������ʵ��ܽ�ȷ����֪ʶ��ֻ���������ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ