��Ŀ����
�����������غ㶨�ɵ����֪ʶ�����ա�
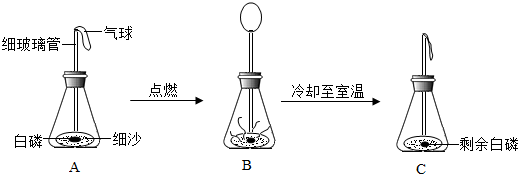
(1)ij���ʸ���������ǿ�ȷ����ֽⷴӦ������CuO��H2O��CO2���ɴ��ƶϸ������к���_________________Ԫ�أ�
(2)CO2�׳ơ�����ɱ�֡���������x���Բⶨ������CO��Ⱦ�ij̶ȣ�x��CO��Ӧ�Ļ�ѧ����ʽΪX+5CO==I2+5CO2���������ɵ�CO2�������ɲⶨCO������������д��X�Ļ�ѧʽ_________________��
(3)����a g������ط�Ӧһ��ʱ�����ʣ�����ʵ�����Ϊbg��������������������_________________��
(1)Cu��H��O��C (2)I2O5 (3)(a�Db)g

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
ͨ�����֡�ͼ�������ݻ�ȡ��Ϣ���Ӷ������ó�ij�ֽ��ۣ��ǽ��л�ѧѧϰ����Ҫ��һ���������±���¼������һ���ܱ������У�A��B��C��D����������һ�������·�����ѧ��Ӧǰ���õ��й����ݣ�
��1���������ݱ�����B���ʿ�����û�ѧ��Ӧ�أ�Ҳ�����Ǹ÷�Ӧ�� ����
��2�����ݱ��е����ݣ����������غ㶨���Ʋ⣬��Ӧ��A���ʵ�����x= g���ɴ˿�֪��һ��Ӧ���� ��Ӧ��
��3�����н������Ѿ�ѧϰ�����ֻ����Ļ�ѧ��Ӧ���ͣ����ϱ�����ʾ�ķ�Ӧ�����⣬���ٸ�дһ����ˮ�����������෴Ӧ�Ļ�ѧ����ʽ���� ���� ���� ��
| ���� | A | B | C | D |
| ��Ӧǰ���� ��g�� | 10 | 2 | 19 | 2 |
| ��Ӧ��������g�� | x | 2 | 2 | 11 |
��2�����ݱ��е����ݣ����������غ㶨���Ʋ⣬��Ӧ��A���ʵ�����x=
��3�����н������Ѿ�ѧϰ�����ֻ����Ļ�ѧ��Ӧ���ͣ����ϱ�����ʾ�ķ�Ӧ�����⣬���ٸ�дһ����ˮ�����������෴Ӧ�Ļ�ѧ����ʽ����