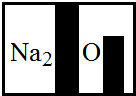��Ŀ����
��ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��������ʾװ���ǻ�ѧС��ͬѧ��Ƶ�ʵ�飮
��1����������������
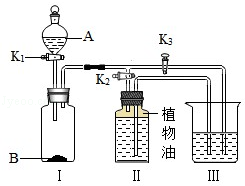
��ͼ1��ʾװ�ã�ͨ����ʵ��֤������������������ ���õĽ����
��2���о�ȼ�յ�������
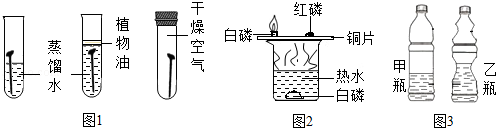
װ����ͼ2��ʾ��ͨ���Ա��� ��������˵����ȼ��ȼ��Ҫ�������Ӵ���
��3���о�������̼���������Ƶķ�Ӧ��
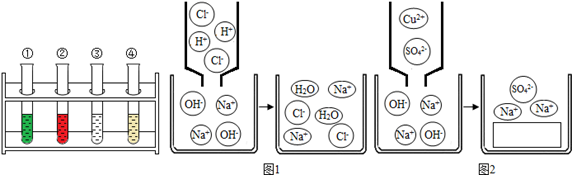
�����������������ҳ���������̼��500mL��Ȫˮƿ�У��ֱ�ע��������ˮ����������Ũ��Һ�����������ͼ3��ʾ����ʵ�����˵��������̼���������Ʒ����˷�Ӧ���������� ����Ϊ��һ��֤��������̼���������Ʒ����˷�Ӧ��ͬѧ�ֲ�����ʵ�飺��ע��������ƿ��ע��������ϡ���ᣬ�۲쵽���������� ������Ӧ�Ļ�ѧ����ʽΪ�� ����

��1����������������
��ͼ1��ʾװ�ã�ͨ����ʵ��֤������������������ ���õĽ����
��2���о�ȼ�յ�������
װ����ͼ2��ʾ��ͨ���Ա��� ��������˵����ȼ��ȼ��Ҫ�������Ӵ���
��3���о�������̼���������Ƶķ�Ӧ��
�����������������ҳ���������̼��500mL��Ȫˮƿ�У��ֱ�ע��������ˮ����������Ũ��Һ�����������ͼ3��ʾ����ʵ�����˵��������̼���������Ʒ����˷�Ӧ���������� ����Ϊ��һ��֤��������̼���������Ʒ����˷�Ӧ��ͬѧ�ֲ�����ʵ�飺��ע��������ƿ��ע��������ϡ���ᣬ�۲쵽���������� ������Ӧ�Ļ�ѧ����ʽΪ�� ����
�ʴ�Ϊ����1��ˮ����������2��ͭƬ�ϵİ���ȼ�ա�ˮ�µİ��ײ�ȼ�գ���3������ͬ�ij��������������̼������ƿ�У�ע������������������Һ������ƿ��ע������ˮ������ƿ�ı��̶ȴ�˵��ע������������Һ��ƿ�ӱ��ֻ�Ƕ�����̼����ˮ���µģ����ж�����̼���������Ʒ����˷�Ӧ��������ð������Ȫˮƿ��ģ�Na2CO3+2HCl�T2NaCl+H2O+CO2����
�����������1��������������������ˮ�Ĺ�ͬ���õĽ������2��ͨ���Ա�ͭƬ�ϵİ���ȼ�ա�ˮ�µİ��ײ�ȼ�յ�����˵����ȼ��ȼ��Ҫ�������Ӵ�����3������ͬ�ij��������������̼������ƿ�У�ע������������������Һ������ƿ��ע������ˮ������ƿ�ı��̶ȴ�˵��ע������������Һ��ƿ�ӱ��ֻ�Ƕ�����̼����ˮ���µģ����ж�����̼���������Ʒ����˷�Ӧ��������̼���������Ʒ�����Ӧ����̼���ƣ�̼���ƺ�ϡ���ᷴӦ���ɶ�����̼���壬�����ע��������ƿ��ע��������ϡ���ᣬ���۲쵽������ð������Ȫˮƿ��ģ���˵��������̼���������Ʒ����˷�Ӧ��

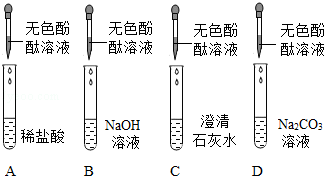
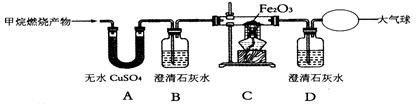
��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�
�����Ŀ