��Ŀ����
ʵ�������и�����ء�ϡ���ᡢ����ʯ������������Һ������������
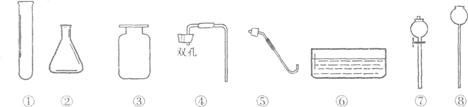
(1)��������ҩƷ��������ȡij���壬��Ҫ�������� (�����)����ȡ�����巴
Ӧ�Ļ�ѧ����ʽΪ ������ʵ��װ��ͼ������ͼ�����ڡ�
(2)��Ҫ��ȡ���﴿�����������壬���Ƚ�����ͨ��ʢ�� ��ϴ��ƿ����ͨ��ʢ��
��ϴ��ƿ��
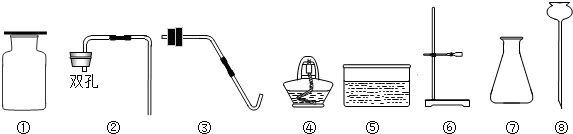
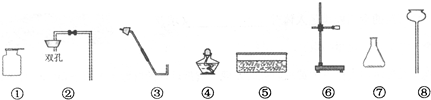
(3)����Ҫ������������������CO2 ��װ�õ����������ã�����©���ͽ��������Ӻá�������������£�
A������ʯ��ˮ��B������������ C������ϡ���D�����˴���ʯ��
��ȷ�IJ���˳��Ϊ
��1���ڢۢܢ� CaCO3+2HCl=CaCl2+H2O+CO2
װ��ͼ����ͼ��2�֣�����װ�ú��ռ�װ�ø�1�֣���һ����1�֣�ע�⣺����©����Һ�治�ܸ�����ƿ��Һ�棩��ע�⣺����©����Һ�治�ܸ�����ƿ��Һ�棩
��2����������Һ��(����)̼��������Һ �� Ũ����
��3��DBAC����ADBC��DABC��


��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ