��Ŀ����
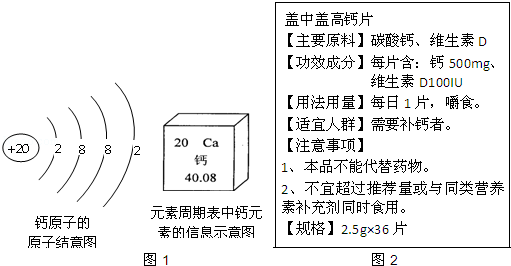
ij��Ƭ�ı�ǩ����ͼ��ʾ����֪�˸�Ƭ�ɷ���ֻ��̼��ƺ��и�Ԫ�ء�
��ͨ������˵���˱�ǩ�еĺ������Ƿ����
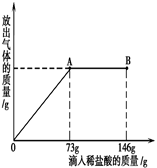
(2)Ϊ�ⶨ����ʵ�ĺ�������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3+2HCl��CaCl+H2O��CO2������ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ�飬�������£�
������ʽ����ÿƬ�˸�Ƭ��̼��Ƶ�������
������ʽ����ÿƬ�˸�Ƭ�ĺ������������鳧����θı�ǩ��
| ִ�б���GB1413��99 ��Ҫ�ɷ֣�̼��� ��������ÿƬ����0.75g ÿƿ50Ƭ����40 g (�������xx��˾��Ʒ) |
(2)Ϊ�ⶨ����ʵ�ĺ�������С��ÿ��ȡ10Ƭ��Ƭ�����ѳ����ĺ�����������ձ��У������Ļ�ѧ��Ӧ�ǣ�CaCO3+2HCl��CaCl+H2O��CO2������ַ�Ӧ���ٳ�ȡ�ձ���ʣ�������������С����������ʵ�飬�������£�
| ���ʵ����� | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| ��Ӧǰ���ձ�+���� | 22 g | 22 g | 22 g | 22 g |
| 10Ƭ��Ƭ | 8 g | 8 g | 8 g | 8 g |
| ��Ӧ���ձ�ʮʣ���� | 26.7 g | 26.5 g | 26.9 g | 26.7g |
������ʽ����ÿƬ�˸�Ƭ��̼��Ƶ�������
������ʽ����ÿƬ�˸�Ƭ�ĺ������������鳧����θı�ǩ��
���������(1)�ȼ����̼��ƣ�CaCO3���и�Ԫ�ص������������ټ����ÿƬ��Ƭ�к��и�Ԫ�ص�������������ǩ�еĺ�������Ƚϣ��жϴ˱�ǩ�еĺ������Ƿ����
��Ʒ��Ϊ��̼��ƣ���Ԫ�ص���������Ϊ
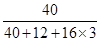 ��100%=40%����ÿƬ������=
��100%=40%����ÿƬ������=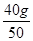 ��40%=0.32g����˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ���������
��40%=0.32g����˱�ƷÿƬ�����ܺ���0.75g���˱�ǩ�еĺ�����������2�����ٸ������⣬��������غ㶨�ɿ�֪����Ӧǰ�ձ��ͷ�Ӧ��������ܺ�-��Ӧ���ձ���ʣ���������=���ɶ�����̼�����������ʹ������ʵ���ƽ��ֵ����Ȼ�����̼��������ᷴӦ�Ļ�ѧ����ʽ������μӷ�Ӧ��̼��Ƶ���������10Ƭ��Ƭ�������������������ÿƬ�˸�Ƭ��̼��Ƶ�������
�⣺��Ӧ���ɵĶ�����̼���������=22g+8g-26.7g=3.3g��
��10Ƭ��Ƭ��̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 ��44
x 3.3g
100��44 =x��3.3g
��ã�x=7.5g
���ÿƬ�˸�Ƭ��̼��Ƶ�����Ϊ7.5g��10=0.75g��
�ڸ���ÿƬ�˸�Ƭ��̼��Ƶ������������ÿƬ�˸�Ƭ�и�Ԫ�ص�������Ȼ��������ı�ǩ�ĺ������顣
ÿƬ�˸�Ƭ�ĺ�����Ϊ0.75g��40%=0.3g��
���ԣ�Ӧ���鳧�ҽ���������ÿƬ����0.75g����Ϊ��ÿƬ����0.3g����
�����������ǹ��ڻ�ѧ����ʽ�ļ����⣬��Ҫ������ͼ������Ӧ����ʽ�������ͽ����ѧ�����е��й����⣬Ҫ��ѧ���н�ǿ�����ݷ�������������Ĺؼ������ñ����ҳ���Ӧ���ɵĶ�����̼����������������صĻ�ѧ��Ӧ����������֪����δ֪��Ӧ�������������㼴�ɡ�

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
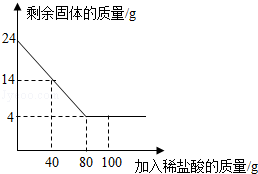
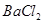 �Ĺ�������49.3g������167g����ˮ����ȫ�ܽ����û����Һ����μ���142g��
�Ĺ�������49.3g������167g����ˮ����ȫ�ܽ����û����Һ����μ���142g��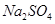 ��Һ��ǡ�÷�Ӧ����
��Һ��ǡ�÷�Ӧ����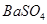 ����������Ϊ23.3g������ʾ��BaCl2+Na2SO4=BaSO4��+2NaCl��������ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�
����������Ϊ23.3g������ʾ��BaCl2+Na2SO4=BaSO4��+2NaCl��������ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�