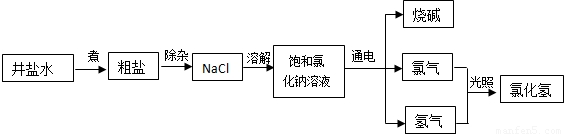
��Ŀ����
 ��2011?��ɽ�����þ��κͺϳɰ������İ�����������̼��������ġ������Ƽ�������ҹ�������ѧ�Һ�°����������ģ����Ȼ��Ƶ������ʸߴ�96%����ԭ����������ͼ��ʾ��
��2011?��ɽ�����þ��κͺϳɰ������İ�����������̼��������ġ������Ƽ�������ҹ�������ѧ�Һ�°����������ģ����Ȼ��Ƶ������ʸߴ�96%����ԭ����������ͼ��ʾ����1������������ˮ����ʳ��ˮͨ�백���ɵõ�����ʳ��ˮ�Ͱ�ˮ�Ļ����ˮ����Ϊ����ˮ��������ˮ��ʳ��ˮ���������ն�����̼����ԭ����
����ˮ�Լ���
����ˮ�Լ���
����2��д���Ȼ����Һ����ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3��
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3��
����������ˮ�Լ��ԣ����������ն�����̼���壻
�Ȼ���ܺ��������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͱ�����
�Ȼ���ܺ��������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͱ�����
����⣺��1������ˮ��ʳ��ˮ���������ն�����̼����ԭ���ǰ���ˮ�Լ��ԣ��ʴ�Ϊ������ˮ�Լ��ԣ�
��2���Ȼ���ܺ��������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͱ�������Ӧ�Ļ�ѧ����ʽΪ��
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3����
��2���Ȼ���ܺ��������Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͱ�������Ӧ�Ļ�ѧ����ʽΪ��
2NH4Cl+Ca��OH��2�TCaCl2+2H2O+2NH3����
�����������Ҫ���������̼�����ʣ������ױ��Լ��Ե���Һ���գ�Ҫ���������غ㶨�ɵ�������ȷ����д��ѧ����ʽ��

��ϰ��ϵ�д�
�����Ŀ