��Ŀ����
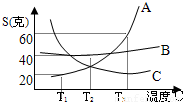
��1������������������в���ȱ�ٵ�һ���л�����Ļ�ѧʽ��C5H10O5���Լ�����ǵ�ʽ���Ƕ��٣���2��A��B��C�������ʵ��ܽ��������ͼ����T1��ʱA���ʵı�����Һ�����ʵ�����������
��3����10��̼��Ƹ�������ϡ���ᷴӦ���ɲ���������̼������ٿˣ�
��4��t°Cʱ��26��п��200��ϡ������Һǡ����ȫ��Ӧ��ǡ�����ɱ�����Һ��
��
�����ɶ��ٿ�������
���ж��ٿ˴�����μӷ�Ӧ��
��t��ʱ����������ܽ���Ƕ��٣���
���𰸡���������1��������Է����������ڸ�Ԫ�����ԭ������֮�ͽ�ɣ�
��2������ij�¶��±�����Һ��������������= ×100%��ɣ�
×100%��ɣ�
��3������̼��Ƶ��������û�ѧ����ʽ�ļ��㼴�ɣ�
��4������п����������п��ϡ����ķ�Ӧ����ʽ�������ɵ����������ͷ�Ӧ������������������������Ӧ����Һ��ˮ������������������ˮ�����������Ӷ�������¶�������п���ܽ�ȣ�
����⣺��1��C5H10O5����Է�������=12×5+1×10+16×5=60+10+80=150��
��2�����ܽ������ͼ��ã�A������T1��ʱ���ܽ��Ϊ20��g����t°Cʱ����Һ��������������= ×100%=16.7%
×100%=16.7%
��3����ɲ���CO2������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
10g x
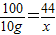
x=4.4g
��4���⣺������H2������Ϊy���μӷ�Ӧ�����������Ϊz�����ɵ�����п������Ϊw
Zn+H2SO4�TZnSO4+H2��
65 98 161 2
26g z w y
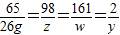
y=0.8g z=39.2g w=64.4g
��ϡ�����к�ˮ��200g-39.2g=160.8g����ˮҲ�Ƿ�Ӧ����Һ���ܼ���������
t��ʱ����������ܽ����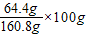 =40.1g
=40.1g
�𣺣�1�����ǵ���Է�������Ϊ150��
��2��T1��ʱ��A���ʵı�����Һ�����ʵ���������Ϊ16.7%��
��3����10��̼��Ƹ�������ϡ���ᷴӦ���ɲ���������̼����4.4g��
��4��������0.8g����������39.2g������μӷ�Ӧ����t��ʱ����������ܽ����40.1g��
����������Ϊ��ѧ����Ŀ����⣬�ص㿼���˻�ѧʽ�ͷ���ʽ�ļ��㣬ͬʱ�漰���ܽ�ȵ���ؼ��㣬���ͻ�������ȫ�棬�ܹ��̳��л�ѧ��ػ��������֪ʶ��
��2������ij�¶��±�����Һ��������������=
 ×100%��ɣ�
×100%��ɣ���3������̼��Ƶ��������û�ѧ����ʽ�ļ��㼴�ɣ�
��4������п����������п��ϡ����ķ�Ӧ����ʽ�������ɵ����������ͷ�Ӧ������������������������Ӧ����Һ��ˮ������������������ˮ�����������Ӷ�������¶�������п���ܽ�ȣ�
����⣺��1��C5H10O5����Է�������=12×5+1×10+16×5=60+10+80=150��
��2�����ܽ������ͼ��ã�A������T1��ʱ���ܽ��Ϊ20��g����t°Cʱ����Һ��������������=
 ×100%=16.7%
×100%=16.7%��3����ɲ���CO2������Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
10g x
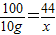
x=4.4g
��4���⣺������H2������Ϊy���μӷ�Ӧ�����������Ϊz�����ɵ�����п������Ϊw
Zn+H2SO4�TZnSO4+H2��
65 98 161 2
26g z w y
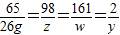
y=0.8g z=39.2g w=64.4g
��ϡ�����к�ˮ��200g-39.2g=160.8g����ˮҲ�Ƿ�Ӧ����Һ���ܼ���������
t��ʱ����������ܽ����
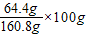 =40.1g
=40.1g�𣺣�1�����ǵ���Է�������Ϊ150��
��2��T1��ʱ��A���ʵı�����Һ�����ʵ���������Ϊ16.7%��
��3����10��̼��Ƹ�������ϡ���ᷴӦ���ɲ���������̼����4.4g��
��4��������0.8g����������39.2g������μӷ�Ӧ����t��ʱ����������ܽ����40.1g��
����������Ϊ��ѧ����Ŀ����⣬�ص㿼���˻�ѧʽ�ͷ���ʽ�ļ��㣬ͬʱ�漰���ܽ�ȵ���ؼ��㣬���ͻ�������ȫ�棬�ܹ��̳��л�ѧ��ػ��������֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
 ��1������������������в���ȱ�ٵ�һ���л�����Ļ�ѧʽ��C5H10O5���Լ�����ǵ�ʽ���Ƕ��٣�
��1������������������в���ȱ�ٵ�һ���л�����Ļ�ѧʽ��C5H10O5���Լ�����ǵ�ʽ���Ƕ��٣�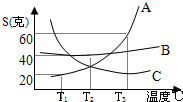 ��1������������������в���ȱ�ٵ�һ���л�����Ļ�ѧʽ��C5H10O5���Լ�����ǵ�ʽ���Ƕ��٣�
��1������������������в���ȱ�ٵ�һ���л�����Ļ�ѧʽ��C5H10O5���Լ�����ǵ�ʽ���Ƕ��٣�