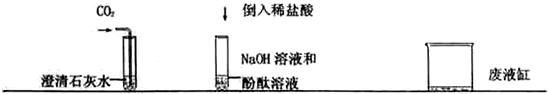
��Ŀ����
СȽ��С����λͬѧ��ʵ�����н��л�ѧʵ�顣СȽ��CO2ͨ������ʯ��ˮ�У�С����ʢ������NaOH��Һ���Թ��е��뼸�η�̪��Һ���ٵ���һ������ϡ���ᡣ

(1)СȽͬѧ�۲쵽������ ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
(2)С��ͬѧʵ������Թ�����ҺΪ��ɫ����Ϊ�������ƺ�����ǡ����ȫ��Ӧ������Ϊ���Ĺ۵��Ƿ���ȷ (��ǡ���)����������ǣ�
��
(3)ʵ�����������ͬѧ����Ӧ���ʣ����ͬʱ�����Һ���У����������ݲ�������Һ��ȻΪ��ɫ�����������ͬѧһ��Է�Һ���з�Һ����ɽ���̽����
�������ԥ����Һ�е����ʳ�ָʾ���⣬����ʲô?
�����в��롿����λͬѧһ����Ϊ����ΪCaCl2��NaCl��
������Ϊ���ʵ���ɻ�����Ϊ ��
��ʵ����֤�����Һ�м��� ������ ����
��ʵ����ۡ�˵������ (��ٻ��)��ȷ��
��ʵ�鷴˼��Ϊ���ٷ�Һ�Ի�������Ⱦ���ɲ�ȡ�ķ����� ��
����չ���졿ͨ����̪��Һ�ɺ�ɫ����ɫ��˵��NaOH����������ȫ��Ӧ������Ϊ��������Ӧ�����Һ�м��� (��ʹ����ɫʯ����Һ��pH��ֽ)��֤��NaOHȷ����ȫ��Ӧ��
(1)��ʯ��ˮ����� CO2+Ca(OH)2=CaCO3��+H2O
(2)�� ��������ʣ�࣬��̪��ҺҲ����ɫ
(3)�����в��롿 CaCl2��NaCl��HCl
��ʵ����֤��п�� �����ݲ���
�ľ�˵����п�� �����ݲ���Ҳ�ɣ�
̼������Һ �����ݲ����Ⱥ����𰸾��ɡ�
��ʵ����ۡ��� �ľ�˵����Ӧ�롾ʵ����֤���еĴ����Ӧ��
��ʵ�鷴˼���������ŷ� �ľ�˵���������۵���ȷ����֡�
����չ���졿����ͭ��Һ �ľ�˵���������ԵĶ���ͭ�εȺ����𰸾���

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�