��Ŀ����
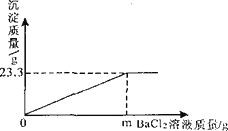
��ͼΪ������Һ���������������ܶȹ�ϵͼ��
��1�����ݸ�ͼ���㽫����������Һ���������������ܶ�֮��Ĺ�ϵ�� _________ ��
��2����Ҫ����100ml������������20%��ϡ���ᣬ��Ҫ������������98%��Ũ���� _________ ml������ʱ��Ӧ�Ƚ�_________���ٽ���һ��Һ�建��ע���ձ��У�����_________��
��3������������װ�������Լ500m1������ˮ��Ũ���ᣨ98%������ͬ�Լ�ƿ����ǩ�����䣩��������ѡ��һ���Լ������û�ѧ�������������ֿ������������ޣ����㽫ѡ�õ��Լ��� _________ ��д���йصĻ�ѧ��Ӧ����ʽ�� _________ ����û���κ�������ҩƷ��Ҫ���������ֳ������㽫��ȡ�ķ����� _________ ��
��1�����ݸ�ͼ���㽫����������Һ���������������ܶ�֮��Ĺ�ϵ�� _________ ��
��2����Ҫ����100ml������������20%��ϡ���ᣬ��Ҫ������������98%��Ũ���� _________ ml������ʱ��Ӧ�Ƚ�_________���ٽ���һ��Һ�建��ע���ձ��У�����_________��
��3������������װ�������Լ500m1������ˮ��Ũ���ᣨ98%������ͬ�Լ�ƿ����ǩ�����䣩��������ѡ��һ���Լ������û�ѧ�������������ֿ������������ޣ����㽫ѡ�õ��Լ��� _________ ��д���йصĻ�ѧ��Ӧ����ʽ�� _________ ����û���κ�������ҩƷ��Ҫ���������ֳ������㽫��ȡ�ķ����� _________ ��
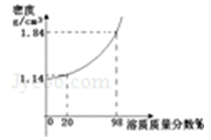
��1��������Һ���ܶ�������Һ�������������������
��2��12.6��ˮ����������
��3��BaCl2��BaCl2+H2SO4==BaSO4��+2HCl�����ֽ���ƿҺ��ֱ����𣬱Ƚ�������С��
��2��12.6��ˮ����������
��3��BaCl2��BaCl2+H2SO4==BaSO4��+2HCl�����ֽ���ƿҺ��ֱ����𣬱Ƚ�������С��

��ϰ��ϵ�д�
�����Ŀ