��Ŀ����
��ͼ�dz��л�ѧ������ʵ��װ�ã�������ѧ��ѧ֪ʶ���ش��й����⣺
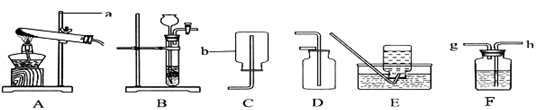
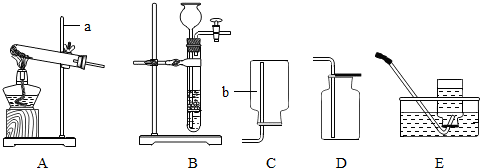
(1)�������ȡװ�ã�
��д�����б�����������ƣ�a_____��b_____��
����KClO��MnO2��ȡO2Ӧѡ�õķ���װ���ǣ�_______ ������ţ���ͬ������ϡ�����п����ȡH2Ӧѡ�õ��ռ�װ����_________��E��
(2)�������ȡԭ������ʵ�����ù��������� Һ�Ͷ������̻����ȡ����,���ж���������___________����,��д����Ӧ�Ļ�ѧ����ʽ��___________��
��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��____________��
(3)����ľ�����
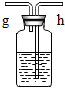
��ʵ����ȡ��O2�к���ˮ��������ʹ��Fװ�ö��������и����װ���ڷ����Һ����_____________������Ӧ�� __________�ˣ�����ĸ��g����h����ͨ�롣
��ʵ ������ ϡ�����п����Ӧ��ȡ�������г�������������HCl���壬Ϊ��ȥH2�е�HCl���壬Fװ���ڷ������Һ��__________��
��д�����б�����������ƣ�a_____��b_____��
����KClO��MnO2��ȡO2Ӧѡ�õķ���װ���ǣ�_______ ������ţ���ͬ������ϡ�����п����ȡH2Ӧѡ�õ��ռ�װ����_________��E��
(2)�������ȡԭ������ʵ�����ù��������� Һ�Ͷ������̻����ȡ����,���ж���������___________����,��д����Ӧ�Ļ�ѧ����ʽ��___________��
��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ��____________��
(3)����ľ�����
��ʵ����ȡ��O2�к���ˮ��������ʹ��Fװ�ö��������и����װ���ڷ����Һ����_____________������Ӧ�� __________�ˣ�����ĸ��g����h����ͨ�롣
��ʵ ������ ϡ�����п����Ӧ��ȡ�������г�������������HCl���壬Ϊ��ȥH2�е�HCl���壬Fװ���ڷ������Һ��__________��
��1���� ����̨ ������ƿ
�� A ��C
��2���� �� ��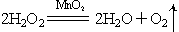
�� CaCO3��2HCl��CaCl2��H2O��CO2��
��3����Ũ���ᣨ��ŨH2SO4����h
���������ƣ���NaOH�ȣ������𰸾��ɣ�
�� A ��C
��2���� �� ��
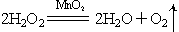
�� CaCO3��2HCl��CaCl2��H2O��CO2��
��3����Ũ���ᣨ��ŨH2SO4����h
���������ƣ���NaOH�ȣ������𰸾��ɣ�

��ϰ��ϵ�д�
�����Ŀ