��Ŀ����
����Ŀ��ʵ������ȡijЩ�����װ����ͼ���ش��й����⡣
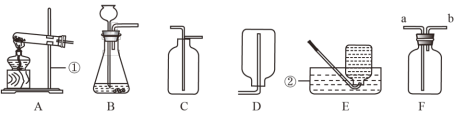
��1��д��װ��ͼ�б�����������ƣ���_____����_____��
��2��д��ʵ����������غͶ���������ȡ�����Ļ�ѧ����ʽ_____����ѡ�õķ���װ����_____������ĸ��������װ��F�ռ��������ɽ������ǵ�ľ������_____����a������b�������ܿڣ����������Ƿ�����
��3����ѡ��װ��B��E�������ȡ�������仯ѧ����ʽ����Ϊ_____����Ҫ���ƽ�ȵ����������ɽ�װ��B�ij���©������_____�����������ƣ�����ѡ��Eװ���ռ�������ԭ����_____��ijͬѧ����װ��E�����ռ�һƿ��100mL���������������Ϊ60%�����壬ʵ��ǰ��Ҫ�ڼ���ƿ��Ԥ��װ��Լ_____mLˮ�������輯��ƿ�е�ˮ���ȫ����ͨ��������ų���
���𰸡�����̨ ˮ��  A b
A b  ��Һ©�� ������������ˮ 50
��Һ©�� ������������ˮ 50
��������
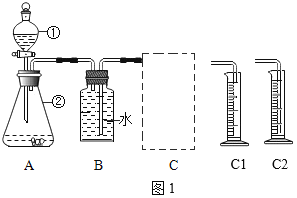
��1��������������̨������ˮ�ۡ�
��2��������ڶ������������������������·�����Ӧ�����Ȼ��ء���������Ӧ�Ļ�ѧ����ʽΪ�� �����ȹ�����ȡ����ѡ����װ��A����ʵ����������غͶ���������ȡ����ѡ�õķ���װ����A������װ��F�ռ������������������ܶȱȿ������ܶȴ�������a�˽��룬������b�˵������ʿɽ������ǵ�ľ������b���ܿڣ����������Ƿ�����
�����ȹ�����ȡ����ѡ����װ��A����ʵ����������غͶ���������ȡ����ѡ�õķ���װ����A������װ��F�ռ������������������ܶȱȿ������ܶȴ�������a�˽��룬������b�˵������ʿɽ������ǵ�ľ������b���ܿڣ����������Ƿ�����
��3����ѡ��װ��B��E�������ȡ�������ǹ�Һ���·�Ӧ��ȡ�����������ù���������Һ��ȡ��������Ӧ�Ļ�ѧ����ʽΪ�� ����Ҫ���ƽ�ȵ����������ɽ�װ��B�ij���©�����ɷ�Һ©������ͨ����ת�������ƹ���������Һ�ĵμ����ʣ��Ӷ����Ʒ�Ӧ���ʡ���ѡ��Eװ���ռ�������ԭ����������������ˮ��ijͬѧ����װ��E�����ռ�һƿ��100mL���������������Ϊ60%�����壬�����к������������Ϊ��100mL��60%=60mL����ʵ��ǰ��Ҫ�ڼ���ƿ��Ԥ��װ��ԼxmL��ˮ������ƿ�ڿ��������Ϊ��100mL-xmL����100mL-xmL����
����Ҫ���ƽ�ȵ����������ɽ�װ��B�ij���©�����ɷ�Һ©������ͨ����ת�������ƹ���������Һ�ĵμ����ʣ��Ӷ����Ʒ�Ӧ���ʡ���ѡ��Eװ���ռ�������ԭ����������������ˮ��ijͬѧ����װ��E�����ռ�һƿ��100mL���������������Ϊ60%�����壬�����к������������Ϊ��100mL��60%=60mL����ʵ��ǰ��Ҫ�ڼ���ƿ��Ԥ��װ��ԼxmL��ˮ������ƿ�ڿ��������Ϊ��100mL-xmL����100mL-xmL����![]() +xmL=60mL�����x=50��
+xmL=60mL�����x=50��

 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�����Ŀ��ijС��Թ���������Һ��ȡ��������������̽����
��̽��һ��̽����ͬ������H2O2�ֽ����ʵ�Ӱ���С���������ͼ1��ʾװ�ý���ʵ�飬ʵ���д�����Ϊ0.4g��H2O2��Һ��Ϊ20mL��Ũ�Ⱦ�Ϊ10%����C�������ӵ��ܺ���Ͳ���������ռ���50mLˮʱ��ij�����ݣ����������Ƴ��±���
�������� | ����������ʣ�mL��s�� |
�������� | 3.5 |
����ͭ | 4.5 |
����̿ | 5.8 |
��1�����Aװ�������Եķ����ǣ��õ��ɼм�סAװ���ҲེƤ�ܣ����Ϸ������ӣ������м�ˮ���ٵĻ�������_____�������������á�
��2��װ��A�з����ķ�Ӧ��ѧ����ʽΪ_____��C����Ӧѡ��_____������C1������C2����װ�á�
��3����̽��ʵ���У���Ҫ�ⶨ��������_____��
��4����ʵ�����ݿ�֪����ͬ�����£����д����Ĵ�Ч����ǿ��������Ϊ_____��
��5������Ͳ���ռ���50mLˮʱ��H2O2�ֽ�������������_____50mL������������������������������
��̽������̽��MnO2��������H2O2�ֽ����ʵ�Ӱ�죬ͼ2����ʾװ���в�����������������ѹǿ������������ڵ��������£��������������װ����ѹǿ�����ȡ���Ӧ���Ⱥ��Բ��ƣ�

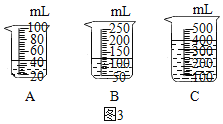
��1��ͼ2������0.1gMnO2�벻ͬ�����3%H2O2��Һ�������ʵ��������ͼ�п��Կ���_____��
��2��������3%H2O2��Һ8�����벻ͬ������MnO2�����ʱ���õ���ͼ����ʾ�����ߡ����ߵ�б����ʾ����MnO2���������ӵ�0.08gʱ��H2O2�ķֽ����ʴﵽʵ��Ҫ���ڴ�ʵ�������£���MnO2������Ϊ4g��һҩ�ף�ʱ����ʹͼ3��_____��ѡ����ĸ���ձ�����װ��3%H2O2��Һ��H2O2�ķֽ�������ӽ�ʵ��Ҫ��
��̽������̽��H2O2��Һ��Ũ�ȶ�H2O2�ֽ����ʵ�Ӱ�죬
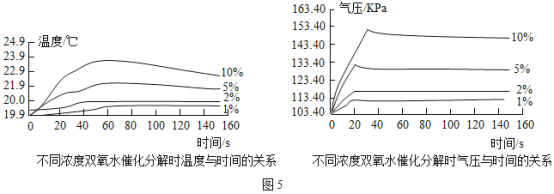
��ͼ4��ʾ����250mL����ƿ�о�����0.5gMnO2���ں�ѹ©���и�����20mL��ͬŨ�ȵ�H2O2��Һ���ֱ����ʵ�顣���¶ȴ��������������Ƴ�ƿ���¶���ʱ��Ĺ�ϵͼ����ͼ5����ʾ���ٽ���װ�õ�����ƿ����ˮԡ���У��óؿ�ʹƿ�ڵ���Һ�¶Ⱥ㶨��20������������ʵ������ͬ���ĸ������ظ�����ʵ�飬����ѹ���������������Ƴ�ƿ����ѹ��ʱ��Ĺ�ϵͼ����ͼ5��ʾ��
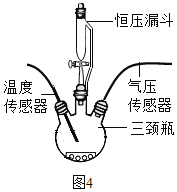
��1��ʵ���У���ѹ©����������_____��
��2����ͼ��֪����H2O2�ֽ�ʱ��_____�����������ų�����������������
��3����ͼ��֪����H2O2��ҺŨ��Խ�ߣ�H2O2�ֽ�����Խ_____������������������������10%H2O2��ҺΪ����Լ25s������ƿ����ѹ�ɸ������͵�ԭ����_____��
����˼���ܽᣩӰ��H2O2�ֽ����ʵ����أ����˴��������ࡢ������������H2O2��Һ��Ũ���⣬��������_____