��Ŀ����
��4�֣�����������������������̼���Ҵ����밴����Ҫ����д��ѧʽ��
(1) ��ɫֲ����й���������յ���_____________
(2)�����ڹ�������ҽ�Ƽ��ȵ���___________
(3)�����ӵ���������Ϊ����ȼ�ϵ���
(4)������ʳƷ��װ�����Է�ʳƷ���ʵ��� ��
��(4��)�����������У���ᷢ�֡���ѧ�������ߡ���
��1��ϴ�ྫ��������ۣ�������������___ __���á�
��2���Ϻ������ڰ�װ�ġ�ֱ��ˮ���������á�����̿+���˲�+�����ߡ���ˮ���ա�����̿�ڴ���__ ___���ã��������ˮ����___ __��ѡ��������������
���ء���ٵ���Ŀ���˶�Ա�ڱ���ǰ���ð�ɫ�ġ�þ�ۡ����֣�������Ϊ��þ�ۡ����ᡢ��ˮ�Ժã���������������þ�ۡ�����Ч�ɷ��Ǽ�ʽ̼��þ��������ȼ��300�漴�ֽ⣬��ֽ�Ļ�ѧ����ʽ�ǣ�Mg5(OH)2(CO3)4 �� 5MgO+X+4CO2��,��X�Ļ�ѧʽ��____
��1��CO2 ��2��O2 ��3��C2H5OH ��4��N2
��1���黯 ��2������ ����� ��3��H2O
��1���黯 ��2������ ����� ��3��H2O
��������1��������̼�ǹ�����õ�ԭ�ϣ�
��2�������ܹ�����������
��3���Ҵ����п�ȼ�ԣ�
��4�������Ļ�ѧ���ʲ����ã�
��1��ϴ�ྫ�����۾����黯���ã�
��2������̿�����������ã�
��3�����ݻ�ѧ����ʽ�����ж����ʵĻ�ѧʽ��
��𣺽⣺��1����ɫֲ����й���������յ��Ƕ�����̼�����CO2��
��2�������ڹ�������ҽ�Ƽ��ȵ������������O2��
��3�������ӵ���������Ϊ����ȼ�ϵ����Ҵ������C2H5OH��
��4��������ʳƷ��װ�����Է�ʳƷ���ʵ��ǵ��������N2��
��1��ϴ�ྫ��������ۣ��������������黯���ã�����黯��
��2������̿�ڴ����������ã��������ˮ���ڻ����������������
��3����Mg5��OH��2��CO3��4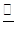 5MgO+X+4CO2����֪��ÿ��X�к���1����ԭ�Ӻ�2����ԭ�ӣ���ˮ�����H2O��
5MgO+X+4CO2����֪��ÿ��X�к���1����ԭ�Ӻ�2����ԭ�ӣ���ˮ�����H2O��
��2�������ܹ�����������
��3���Ҵ����п�ȼ�ԣ�
��4�������Ļ�ѧ���ʲ����ã�
��1��ϴ�ྫ�����۾����黯���ã�
��2������̿�����������ã�
��3�����ݻ�ѧ����ʽ�����ж����ʵĻ�ѧʽ��
��𣺽⣺��1����ɫֲ����й���������յ��Ƕ�����̼�����CO2��
��2�������ڹ�������ҽ�Ƽ��ȵ������������O2��
��3�������ӵ���������Ϊ����ȼ�ϵ����Ҵ������C2H5OH��
��4��������ʳƷ��װ�����Է�ʳƷ���ʵ��ǵ��������N2��
��1��ϴ�ྫ��������ۣ��������������黯���ã�����黯��
��2������̿�ڴ����������ã��������ˮ���ڻ����������������
��3����Mg5��OH��2��CO3��4
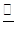 5MgO+X+4CO2����֪��ÿ��X�к���1����ԭ�Ӻ�2����ԭ�ӣ���ˮ�����H2O��
5MgO+X+4CO2����֪��ÿ��X�к���1����ԭ�Ӻ�2����ԭ�ӣ���ˮ�����H2O��
��ϰ��ϵ�д�
�����Ŀ

 ��3���ù������������������������ ����
��3���ù������������������������ ���� ��4��C2H2 + O2 CO2 + H2O
��4��C2H2 + O2 CO2 + H2O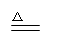 ��5��Fe2O3 + H2 Fe + H2O
��5��Fe2O3 + H2 Fe + H2O
