��Ŀ����
������ߺ�DZˮͧ�п��ù������ƣ�Na2O2����Ϊ��������Ϊ��̽���䷴Ӧԭ������ȤС��ͬѧ����ʦ��ָ���£�����������̽��������һ����롣
[��������]���������ڳ�������ˮ��������̼��Ӧ�ֱ������������ơ�̼���ƺ�������
[���ʵ��]����ͬѧ��ͨ����ͼ��ʾװ��̽�����������������̼�ķ�Ӧ������֤��Ӧ���
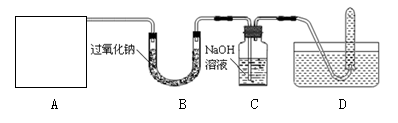
��1��������ʵ���ҳ��õ�����:

��A�Ƕ�����̼����ķ���װ�ã�װ���װ��ʱ��Ӧѡ�õ������������ܵ�˫����Ƥ���⣬����Ҫ�������У������ƣ� �� ��ʵ������ȡ������̼�Ļ�ѧ����ʽ ����װ����ʵ���һ�����������ȡ������д���ø�װ����ȡ�����Ļ�ѧ����ʽ ��
��ʵ����ѡ����ȡ����ķ���װ�ã���Ҫ������ �� ��
��2����D��ʾ�ķ����ռ��������������� ���ռ�����ʱ��������ѡ�� ���ռ�������Dװ���Թ����ռ����е������Ƿ������ķ������������ǣ���D�����弯�����Թ��Ƴ�ˮ�棬Ȼ��
��
��3��C���������Ƶ�����������û�в��뷴Ӧ��CO2�����û���������װ�ã����ܵ��µĺ���� ��
��4������B�з�Ӧ��ʣ�����ijɷ֡�
[��˼������]��1��ͨ������ʵ�飬����ͬѧ��ΪNa2O2��CO2��Ӧ��������Na2CO3��O2�����п�������NaHCO3������Ϊ���Ľ��� ��ѡ���ȷ������ȷ������������ ��д��Na2O2�������̼��Ӧ�Ļ�ѧ����ʽ ��
��˵˵�������DZˮͧ�ù���������Ϊ���������������ŵ� ��
[��������]���������ڳ�������ˮ��������̼��Ӧ�ֱ������������ơ�̼���ƺ�������
[���ʵ��]����ͬѧ��ͨ����ͼ��ʾװ��̽�����������������̼�ķ�Ӧ������֤��Ӧ���

��1��������ʵ���ҳ��õ�����:

��A�Ƕ�����̼����ķ���װ�ã�װ���װ��ʱ��Ӧѡ�õ������������ܵ�˫����Ƥ���⣬����Ҫ�������У������ƣ� �� ��ʵ������ȡ������̼�Ļ�ѧ����ʽ ����װ����ʵ���һ�����������ȡ������д���ø�װ����ȡ�����Ļ�ѧ����ʽ ��
��ʵ����ѡ����ȡ����ķ���װ�ã���Ҫ������ �� ��
��2����D��ʾ�ķ����ռ��������������� ���ռ�����ʱ��������ѡ�� ���ռ�������Dװ���Թ����ռ����е������Ƿ������ķ������������ǣ���D�����弯�����Թ��Ƴ�ˮ�棬Ȼ��
��
��3��C���������Ƶ�����������û�в��뷴Ӧ��CO2�����û���������װ�ã����ܵ��µĺ���� ��
��4������B�з�Ӧ��ʣ�����ijɷ֡�
| ʵ�鲽�� | ʵ������ | ʵ����ۼ�����ʽ |
| ��ȡ����A�з�Ӧ��Ĺ������Թ��У� �� �� �� �� | �� �� | ��Ӧ�����ɵĹ�����̼���ơ�д������۵Ļ�ѧ����ʽ |
[��˼������]��1��ͨ������ʵ�飬����ͬѧ��ΪNa2O2��CO2��Ӧ��������Na2CO3��O2�����п�������NaHCO3������Ϊ���Ľ��� ��ѡ���ȷ������ȷ������������ ��д��Na2O2�������̼��Ӧ�Ļ�ѧ����ʽ ��
��˵˵�������DZˮͧ�ù���������Ϊ���������������ŵ� ��
������17�֣�
��1������ƿ������©����
CaCO3 + 2HCl �� CaCl2 + H2O + CO2����2H2O2 2H2O + O2�� ����2�֣���8�֣�
2H2O + O2�� ����2�֣���8�֣�
�ڷ�Ӧ���״̬����Ӧ�Ƿ���Ҫ���ȣ���Ӧ������ ����1�֣���2�֣�
��2��������������ˮ ��2�֣�
�����ſ��� ��1�֣�
��ȼ�ŵ�ľ�������Թ��У�ľ����ȼ�������������� ��3�֣�
��3��Ӱ�������ļ��飨������������ɣ� ��2�֣�
��4�� �����衢�����1�֣�����ʽ2�֣���6�֣�
[��˼������]��1������ȷ ��1�֣�
��Ӧ����û����Ԫ�أ����������غ㶨�ɣ�����������NaHCO3 ��2�֣�
2Na2O2 + 2CO2�� 2Na2CO3 + O2 ��2�֣�
��2�����պ��������Ķ�����̼��ͬʱ�������� ��2�֣�
��1������ƿ������©����
CaCO3 + 2HCl �� CaCl2 + H2O + CO2����2H2O2
 2H2O + O2�� ����2�֣���8�֣�
2H2O + O2�� ����2�֣���8�֣��ڷ�Ӧ���״̬����Ӧ�Ƿ���Ҫ���ȣ���Ӧ������ ����1�֣���2�֣�
��2��������������ˮ ��2�֣�
�����ſ��� ��1�֣�
��ȼ�ŵ�ľ�������Թ��У�ľ����ȼ�������������� ��3�֣�
��3��Ӱ�������ļ��飨������������ɣ� ��2�֣�
��4�� �����衢�����1�֣�����ʽ2�֣���6�֣�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| �ڵμ�ϡ���� �۽����ɵ�����ͨ�����ʯ��ˮ�� | �������ݲ��� �۳���ʯ��ˮ����� | Ca(OH)2 + CO2�� CaCO3�� + H2O |
[��˼������]��1������ȷ ��1�֣�
��Ӧ����û����Ԫ�أ����������غ㶨�ɣ�����������NaHCO3 ��2�֣�
2Na2O2 + 2CO2�� 2Na2CO3 + O2 ��2�֣�
��2�����պ��������Ķ�����̼��ͬʱ�������� ��2�֣�
����:��1��ʵ����ͨ����̼��ƺ�ϡ���ᷴӦ��ȡ������̼���壮�������������ƿ������©���������ܵ���Ƥ���ȣ�
��2���ռ����������ͨ���Ǹ���������ܶ����ܽ��ԣ�������ˮ�ģ���������ˮ���ռ����ȿ����ܶȴ�ģ��������������ռ����ȿ����ܶ�С�ģ��������������ռ�
��3������������Һ�������ն�����̼���壬������������Һ�Ǽ��������̼�����
��4����������̼���η�Ӧ���ɶ�����̼���壬������̼������ʹ�����ʯ��ˮ����ǣ�
[��˼������]
��1�����������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��Ԫ�ص������
��2�������������������̼��Ӧ����������
�⣺��1��������ͼ��֪��������ȡ����ķ�Ӧ���ǹ����Һ�壬��Ӧ����Ҫ���ȣ��ʴ�Ϊ������ƿ������©����
ʵ������ȡ������̼�Ļ�ѧ����ʽCaCO3+2HCl=CaCl2+H2O+CO2������װ����ʵ���һ�����������ȡ������д���ø�װ����ȡ�����Ļ�ѧ����ʽ2H2O2
 2H2O+O2��
2H2O+O2����ʵ����ѡ����ȡ����ķ���װ�ã���Ҫ�����ǣ���Ӧ���״̬����Ӧ�Ƿ���Ҫ���ȣ���Ӧ��������
��2����D��ʾ�ķ����ռ��������������ǣ�����������ˮ��
��3�����û������������Һ���ն�����̼���壬��D�ռ�������������������ж�����̼���壮�ʴ�Ϊ��Ӱ�������ļ��飨������������ɣ���
��4�������������������̼��Ӧ����̼���ƺ�������Bװ������̼���ƹ��壬���Դ����Ŀ���Ǽ���̼���ƣ�̼��������̼���Σ���������̼���������ᷴӦ���ɶ�����̼���������𣮹ʴ�Ϊ��
| ʵ�鲽�� | ʵ������ |
| �ڵμ�ϡ���� �۽����ɵ�����ͨ�����ʯ��ˮ�� | �������ݲ��� �۳���ʯ��ˮ����� |
2Na2O2+2CO2=2Na2CO3+O2
��2���������DZˮͧ�ù���������Ϊ������������ŵ㣺���պ��������Ķ�����̼��ͬʱ����������
�ʴ�Ϊ����1������ƿ������©����CaCO3+2HCl=CaCl2+H2O+CO2����2H2O2
 2H2O+O2����
2H2O+O2�����ڷ�Ӧ���״̬����Ӧ�Ƿ���Ҫ���ȣ���Ӧ������
��2��������������ˮ����3��Ӱ�������ļ��飨������������ɣ�����4��
| ʵ�鲽�� | ʵ������ |
| �ڵμ�ϡ���� �۽����ɵ�����ͨ�����ʯ��ˮ�� | �������ݲ��� �۳���ʯ��ˮ����� |
��2�����պ��������Ķ�����̼��ͬʱ����������
�������ۺ��Ե�ʵ��̽���⣬�����˶���֪ʶ��̼���εļ����������̼�����������ᷴӦ���ɶ�����̼���壬������̼��ʹ�����ʯ��ˮ����ǵ���һ���ʣ�

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ

 5MgO��5H2O��4CO2����������һ��ѧ��Ӧ�ṩ����Ϣ�����롰þ�ۡ������������һ����;�� �� ��
5MgO��5H2O��4CO2����������һ��ѧ��Ӧ�ṩ����Ϣ�����롰þ�ۡ������������һ����;�� �� ��





 �����ӳ�ٶȣ�
�����ӳ�ٶȣ� 8.��������ͼ����װ����������������ش����⣺
8.��������ͼ����װ����������������ش����⣺

