��Ŀ����
 �±���Ԫ�����ڱ���һ���֣���۲첢���������գ�
�±���Ԫ�����ڱ���һ���֣���۲첢���������գ���1���ؿ��к������������ķǽ���Ԫ��������
��
��
����Ԫ�ص�������Ϊ14
14
����2����12��Ԫ�ص����ӷ���Ϊ
Mg2+
Mg2+
����3����ԭ������Ϊ 1��6��7��8 ������Ԫ����ɵĻ��������ʯ���д̼�����ζ�������ɣ���������Ϊ��һ�۵Ļ����ﻯѧʽ��
NH4HCO3
NH4HCO3
����4�������л��ﶼ����һ��Ԫ�����ڵ�
һ
һ
���ڣ��۲�Ԫ�����ڱ�ͬһ���е�Ԫ�����У��㷢�ֵĹ��������Ӳ�����ͬ��
���Ӳ�����ͬ��
��дһ�㼴�ɣ�����5��Ũ��ˮ��Ũ��������д������̣�����Ϊ���߾���
�ӷ���
�ӷ���
�����������ʣ����������Ȼ����������а�ɫ������������һ���Σ�д���䷽��ʽNH3+HCl=NH4Cl
NH3+HCl=NH4Cl
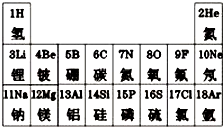
����������1�����ݵؿ��и�Ԫ�غ�������
��2���������ӷ��ŵ���д������д
��3�����ݻ�ѧʽ����д������д
��4���������ڱ����ص����
��5������Ũ��ˮ��Ũ�������������ѧ���ʷ���
��2���������ӷ��ŵ���д������д
��3�����ݻ�ѧʽ����д������д
��4���������ڱ����ص����
��5������Ũ��ˮ��Ũ�������������ѧ���ʷ���
����⣺��1���ؿ��к����ɸߵ��͵�˳��ǰ��λ��Ԫ��Ϊ�����衢�����������������ķǽ���Ԫ�������ǹ裬��Ԫ�ص�������Ϊ14��
��2����12��Ԫ��ΪþԪ�أ�����������������ӣ���ʧȥ�������ӳ�Ϊþ���ӣ���þ���ӷ���ΪMg2+��
��3��ԭ������Ϊ 1��6��7��8 ������Ԫ�طֱ�Ϊ�⡢̼������������ɵĻ�����̼���������ʯ���д̼�����ζ�İ������ɣ���������̼���������Ϊ��һ�ۣ��ʻ�����Ϊ̼����泥���ѧʽΪNH4HCO3��
��4�������л��ﶼ������Ԫ�أ����ڵ�һ���ڣ�Ԫ�����ڱ�ͬһ���е�Ԫ����ԭ�Ӻ��ⶼ����ͬ�ĵ��Ӳ�����
��5��Ũ��ˮ��Ũ���ᶼ�лӷ��ԣ������ʱ��Ũ��ˮ�ӷ����İ�����Ũ����ӷ������Ȼ�����Ӳ����˶���������һ������ѧ��Ӧ�����Ȼ�藺�ɫ����С����������д������̣��䷴Ӧ�Ļ�ѧ����ʽNH3+HCl=NH4Cl
�ʴ�Ϊ����1���裬14��
��2��Mg2+��
��3��NH4HCO3��
��4��һ�������Ӳ�����ͬ
��5���ӷ��� NH3+HCl=NH4Cl
��2����12��Ԫ��ΪþԪ�أ�����������������ӣ���ʧȥ�������ӳ�Ϊþ���ӣ���þ���ӷ���ΪMg2+��
��3��ԭ������Ϊ 1��6��7��8 ������Ԫ�طֱ�Ϊ�⡢̼������������ɵĻ�����̼���������ʯ���д̼�����ζ�İ������ɣ���������̼���������Ϊ��һ�ۣ��ʻ�����Ϊ̼����泥���ѧʽΪNH4HCO3��
��4�������л��ﶼ������Ԫ�أ����ڵ�һ���ڣ�Ԫ�����ڱ�ͬһ���е�Ԫ����ԭ�Ӻ��ⶼ����ͬ�ĵ��Ӳ�����
��5��Ũ��ˮ��Ũ���ᶼ�лӷ��ԣ������ʱ��Ũ��ˮ�ӷ����İ�����Ũ����ӷ������Ȼ�����Ӳ����˶���������һ������ѧ��Ӧ�����Ȼ�藺�ɫ����С����������д������̣��䷴Ӧ�Ļ�ѧ����ʽNH3+HCl=NH4Cl
�ʴ�Ϊ����1���裬14��
��2��Mg2+��
��3��NH4HCO3��
��4��һ�������Ӳ�����ͬ
��5���ӷ��� NH3+HCl=NH4Cl
������������Ԫ�����ڱ�Ϊ���壬�����˵ؿ���Ԫ�ء����ӷ��š���ѧʽ����ѧ����ʽ����д���Լ�Ԫ�����ڱ����ص㣬�ۺ��Խ�ǿ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
�±���Ԫ�����ڱ���һ���֣�
��1��16��Ԫ�ص�Ԫ�ط���Ϊ ����Ԫ�ص�ԭ�ӽṹʾ��ͼ���ң���X����ֵ= ��
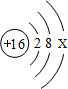
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ ��
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ�� ��
| �� ���� |
IA | 0 | ||||||
| һ | 1H 1��008 |
��A | ��A | ��A | V A | ��A | ��A | 2He 4��003 |
| �� | 3Li 6��941 |
4Be 9��012 |
5B 10��81 |
6C 12��01 |
7N 14��01 |
8O 16��00 |
9F 19��00 |
10Ne 20��18 |
| �� | 11Na 22��99 |
12Mg 24��31 |
13Al 26��98 |
14Si 28��09 |
15P 30��97 |
16S 32��06 |
17Cl 35��45 |
18Ar 39��95 |
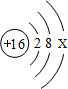
��2��8��Ԫ�غ�13��Ԫ����ɵĻ�����Ļ�ѧʽΪ
��3�����ñ��е�Ԫ�ط�������ɵķ�Ӧ��д������Ҫ��Ļ�ѧ����ʽ����ˮ���ɵĻ��Ϸ�Ӧ��


 ����m=
����m=
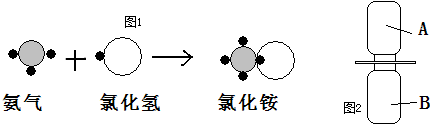
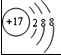 ��ʾ����
��ʾ���� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ��á�
����ʾ��ԭ�ӣ��á� ����ʾ��ԭ�ӣ�������Ӧ���̿���ͼ1��ʾ��
����ʾ��ԭ�ӣ�������Ӧ���̿���ͼ1��ʾ��